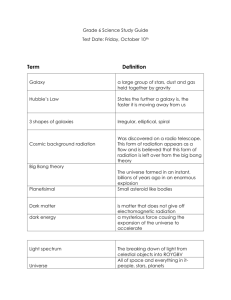
Commentary to support the PowerPoint on Course
advertisement
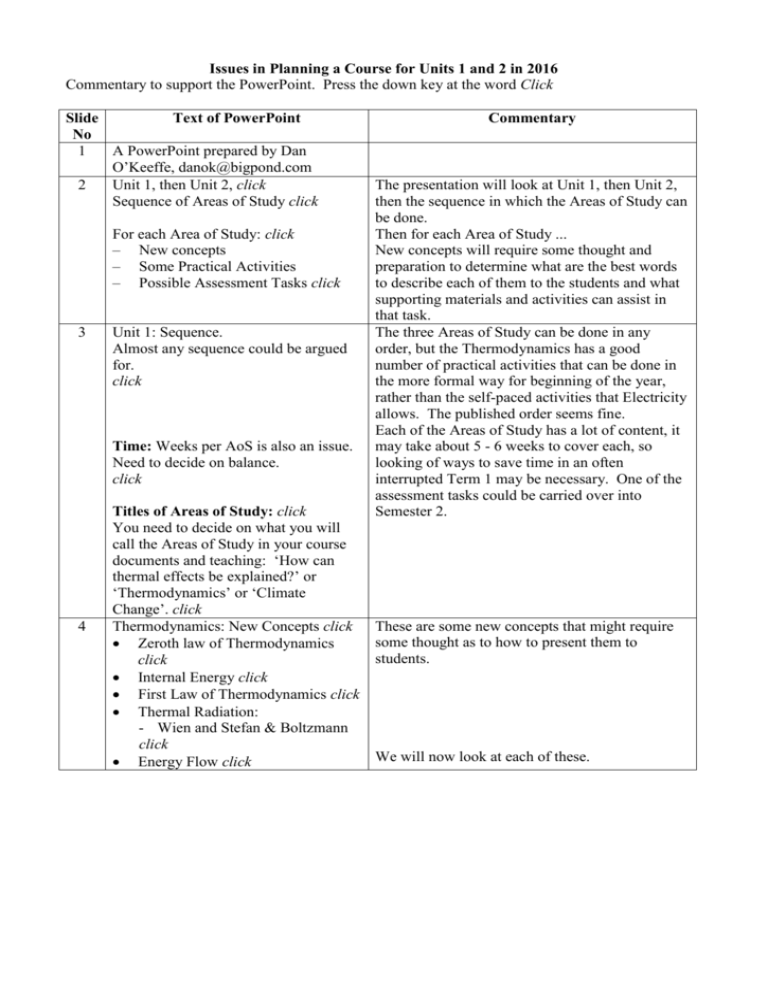
Issues in Planning a Course for Units 1 and 2 in 2016 Commentary to support the PowerPoint. Press the down key at the word Click Slide Text of PowerPoint No 1 A PowerPoint prepared by Dan O’Keeffe, danok@bigpond.com 2 Unit 1, then Unit 2, click Sequence of Areas of Study click For each Area of Study: click – New concepts – Some Practical Activities – Possible Assessment Tasks click 3 Unit 1: Sequence. Almost any sequence could be argued for. click Time: Weeks per AoS is also an issue. Need to decide on balance. click 4 Titles of Areas of Study: click You need to decide on what you will call the Areas of Study in your course documents and teaching: ‘How can thermal effects be explained?’ or ‘Thermodynamics’ or ‘Climate Change’. click Thermodynamics: New Concepts click Zeroth law of Thermodynamics click Internal Energy click First Law of Thermodynamics click Thermal Radiation: - Wien and Stefan & Boltzmann click Energy Flow click Commentary The presentation will look at Unit 1, then Unit 2, then the sequence in which the Areas of Study can be done. Then for each Area of Study ... New concepts will require some thought and preparation to determine what are the best words to describe each of them to the students and what supporting materials and activities can assist in that task. The three Areas of Study can be done in any order, but the Thermodynamics has a good number of practical activities that can be done in the more formal way for beginning of the year, rather than the self-paced activities that Electricity allows. The published order seems fine. Each of the Areas of Study has a lot of content, it may take about 5 - 6 weeks to cover each, so looking of ways to save time in an often interrupted Term 1 may be necessary. One of the assessment tasks could be carried over into Semester 2. These are some new concepts that might require some thought as to how to present them to students. We will now look at each of these. 5 6 Zeroth law of Thermodynamics A late addition to physics, for completeness click A definition of temperature. click “All heat is of the same kind” click Internal Energy click You are heating a substance, what happens to its atoms and molecules? click Gas: Monatomic: Ne click Diatomic: O2 click Multi atom: H2O click Liquid: Solid: click Atoms and molecules have different types of energy. click Only translational kinetic energy relates to Temp. click This is an unusual addition to this Area of Study. It is quite a subtle point. The Zeroth Law was introduced in the 20th Century so that the concept of 'Thermal equilibrium' was an 'equivalence relation' in the mathematical and logical sense. The statement in the study design is an oversimplification of the Zeroth law. Nevertheless its significance is subtle and there is little that students can do with it other than know it, so it probably needn't be given much class time with Year 11 students. It may be enough to just say that is provides a definition of temperature. This is what Maxwell thought on the subject. The graphic is the real Zeroth Law, that is, if A is in thermal equilibrium with B and with C, then B and C are also in thermal equilibrium with each other. This is the transitive condition of equivalence. click Internal Energy is an important concept. It explains why the temperature stays the same while ice melts and why specific heat capacities vary so much. It is also mentioned in a quantitative sense, so it deserves some explanation. Let's consider gases and solids, liquids have features of both. What happens to the atoms if you heat Neon gas? Ans: The atoms move What happens to the molecules if you heat Oxygen? Ans: They move, but what else can a diatomic molecule do? Ans: They spin and stretch What happens to the molecules if you heat water vapour? Ans: They move, spin, stretch and what else? Ans: They twist All these actions have a kinetic energy related to it. For a solid, the atoms can move, but they are constrained by attractive and repulsive forces. So the forms of energy include: rotational kinetic energy, vibrational kinetic energy, torsional kinetic energy, translational kinetic energy and potential energy in the case of solids. So, which motion or energy is related to temperature? Note: The study design is inexact, if not wrong. Note: Water has a very high specific heat capacity because the water molecule has so many ways of putting the energy away without increasing the temperature. Note: If the heat capacities of He, Ne and Ar are multiplied by their respective atomic weights, you get the same answer, meaning, if you added 100 joules of energy to a mixed gas of 100 atoms, each atom would receive 1 joule of energy regardless of its mass. 7 8 9 10 11 12 13 Animations of modes of vibration of water and carbon dioxide molecules. click 1st Law of Thermodynamics click Energy is conserved, click but done quantitatively and best with a gas example click Three terms: click • Energy can be added to a system, Q • Work can be done by a system, W • Internal energy can change as a result, U • U = Q – W click Only simple calculations. click Radiation and Greenhouse Effect click A significant section with many new physics concepts: click • Spectrum click • Temperature means click • atoms jiggle click • electrons jiggle click • accelerating charges click • electromagnetic radiation click • Freq, wavelength click • Energy, power click Radiation and Greenhouse Effect click Increased temperature means click • atoms jiggle faster click • electrons jiggle faster click • higher frequency radiation click • higher energy click Wien’s Law: How does the wavelength of maximum intensity vary with temperature? click maxT = constant click Stefan-Boltzmann Law: How does total energy emitted vary with temperature? Consider Area under the graph: Power ∝ T4. click What determines the Earth’s surface temperature? Light from Sun click Light reflected by Earth click These modes are relevant when considering climate change. This is a basic enough concept, but ... There are three terms, note the words 'to' and 'by' are important to get the sign right. Note: The study design has left out the in U and written the relationship in an unconventional way. This is how most text books write it. This can be a complicated topic, in practice Q, W and U can be positive or negative, so stay with simple problems, e.g. A gas is heated by 200 joules of energy, the gas expands doing 50 joules of work. What is the change in internal energy? The em spectrum is an important part of this Area of Study. Students need to know the different types of radiation and some sense of how they vary in frequency and wavelength. But they also need an explanation of why hot objects give off light. Which means .. ditto ditto ditto ditto They should also have and understanding of these terms without being quantitative. Self explanatory Students need to be familiar with the shape of these graphs, realise that the peak wavelengths follow an inverse curve with temperature. They only need to know the relationship with constant, so you could limit the problems to calculation by ratio, but the actual value should not present too many problems for most students. Ratio problems only. The actual equation is for teacher reference only. The average over 24 hours and from pole to pole is 340 W/m2, 100 W/m2 is reflected back by clouds and ice leaving ...? 14 15 16 17 18 19 20 21 22 23 Light from the Sun heats the Earth ... click click How hot must the Earth be to radiate 240 W/m2 ? click What determines the Earth’s surface temperature? click click The Earth’s surface is 33 °C warmer than it would be if it had no atmosphere. So how does the atmosphere warm the Earth’s surface? click Nitrogen (N2), Oxygen (O2),Argon (Ar) • More than 99% of the atmosphere • These molecules have one or two atoms • They block some ultra-violet light, but • Allow infra-red and visible radiation through. click With an atmosphere of Nitrogen Oxygen & Argon, what would be the surface temperature? click click Greenhouse warming is caused by Water (H2O), Carbon Dioxide (CO2) click What is special about H2O and CO2? • Their molecules have three atoms, • Their natural frequencies of vibration are in the infra-red, • They are the earth’s blanket for reflecting certain infra-red frequencies back down to earth click Infrared Radiation absorbed by Water and CO2 click Radiation from the earth. click Absorption spectrum of water. click Water's absorption spectrum now overlays the spectrum of what the earth is emitting. click Absorption spectrum of CO2. click CO2's absorption spectrum now overlays the spectrum of what the earth is emitting. click Greenhouse Warming click 240 W/m2 to heat the earth and at thermal equilibrium, the warmed earth to radiate 240 W/m2. This should be a surprising result. Why is the earth's surface 33 degrees hotter than it needs to be? Ans: Greenhouse effect The key feature is that they are transparent to infra red, so ... Not surprisingly, it would still be -180C The modes of vibration you saw earlier are the ones that absorb and then re-radiate infra red, but in re-radiating some goes up and some comes down. We start with the em spectrum and the shape of the radiation coming from the sun. Next comes the Earth's spectrum. Up comes the absorption spectrum of water. The dark areas are the wavelengths that water absorbs. Where the graph for water overlaps, these wavelengths are absorbed then re-radiated up and down. Up comes the absorption spectrum of CO2. Different wavelengths of the earth's emission are absorbed. Water contributes more, but CO2 stays around much longer. 24 Diagram on Energy Flow from IPCC click 25 Introductory activities on phenomena to stimulate curiosity and generate students’ questions Experiments Heat capacity: i) mixing liquids, ii) adding heated block to water iii) heat capacity of thermos iv) microwave oven expt 1st Law:Calorimeter prac (link to Elec) Latent Heat: i) Add ice to hot water Mechanical Equivalent of heat click Experiments ctd: • Absolute Zero from Volume vs Temp Radiation: • Spectra of hot objects, • Stefan-Boltzmann Expt Investigation: • Keeping it Hot – design, build & test Discount craft supplies Reverse Art Truck Spreadsheet: Investigation of a Climate model click Assessment tasks could be: • Written response to a selection of context questions • Exploration of an issue related to thermodynamics click 26 27 This IPCC images summarises the energy flow. Although note that the units are Watts per square metre. Each of the entries is an energy transfer, so students can determine which process is involved: conduction, etc. An energy accounting exercise can be down in three locations: i) the earth as a whole (342 = 235 + 107), ii) just the energy entering and leaving the Earth's surface and also iii) the energy entering and leaving the atmosphere. A 'what if' exercise can also be done. If the Arctic Sea ice decreases and the 30 W/m2 reflected reduces to 25, what happens? The earth absorbs more energy, increases in temperature, radiates out more, some of which is re-emitted back and absorbed, etc until a new higher thermal equilibrium is reached. This can be modeled with a simple spreadsheet. Introductory activities: a series of short tasks, such as dabbing metho on wrist to observe cooling by evaporation,etc. See http://www.vicphysics.org/thermodynamics.html for examples of activities and questions. The students' questions can form the basis of an assessment task later in the topic. Calorimeter possibly done in Electricity. Students are given a plastic cup and are asked to design a 'thermos' using materials such as foam, fabric, rubber, alfoil, masking tape, etc. Then the next day build the design and test it with thermometer or temp probe. Then write it up. Students' questions from introductory task. Specified in the study design with seven issues listed. 28 Exploration of an issue Apply thermodynamic principles to investigate at least one issue related to the environmental impacts of human activity with reference to the enhanced greenhouse effect. click Consider: other topics such as solar thermal power, click geo-engineering, blog related discussion .... click integrating the task into the work program. click a team approach with a group presentation. click 29 30 how much resourcing do you supply. click how much guidance and structure click Electricity Extra Content Voltage dividers Specific reference to thermistors, LDRs, LEDs Energy transfer with reference to transducers Specific reference to Residual Current Devices So, basically the same, with slightly extra content, which many currently do. click What is Matter? Origins of atoms click Big Bang and Cosmology click Particles in the nucleus Radioactivity and Nuclear forces click Hadrons and Leptons, Baryons and Mesons, Quarks click Anti-matter click Energy from the atom Fission and Fusion click Binding energy and E = mc2 click Production of light click How to group the content? In what order do you want to teach it? click Some aspects to consider are: There are other issues that are also interesting to students. Rather than doing the task at the end of the topic, students could be given the task earlier, so it is done mostly as homework, but with occasional monitoring. A team approach may reduce the workload on the students and all students would benefit from various presentations. These are always questions of balance. Very little change, many teachers already do Voltage dividers in Year 11, so no need to elaborate on this Area of Study further. This is a summary of the topics in this Area of Study as presented in the study design. The task for you is to decide: do you want to group then differently e.g. follow radioactivity with fission and fusion? and in what order do you want to cover the content? The study design begins at the beginning of the universe, an alternative is .... 31 32 33 34 Different approaches are possible. History of Science view: Radioactivity: decay, half life, nuclear transformations, decay series as well as b+ and neutrino. Fission and Fusion: Equations, Binding energy and E = mc2. Discovery of extra particles: anti-particles, hadrons, then mesons and baryons leading to quarks. click History of Science view ctd: Cosmology: Big Bang theory including inflation, elementary particle formation, annihilation of anti-matter and matter, commencement of nuclear fusion, cessation of fusion and the formation of atoms. Production of light: accelerating charges, synchrotron, energy level transition click Challenges click Most of the new stuff!, however … click It is mostly descriptive, so …. click Treat it to your own comfort level, e.g. click Cover cosmology with a 50 min Brian Cox video, or Applets from The Particle Adventure, CERN, click New concepts: Anti-matter: Introduce beta plus decay with beta minus decay. click Neutrino: Introduce to explain energy discrepancy in beta decay.click Forces: Strong and weak click Muon, etc: This approach is similar to that for the current topic, starting with Becquerel and progressing through the 20th century as discoveries are made and explanations change. The inclusion of 'inflation' makes this a more complex topic as the inflation model addressed the inadequacies of the big band model. Similarly, the explanations for different modes of producing light can be quite complex, so care is needed in how these are approached with Year 11 students. There is a lot for students to recall, but it is not quantitative. Take it to the depth you want to. There are plenty of video resources available that can do the job for you Compare range, strength and what they explain Alpha spectra is discrete The presence of lines in atomic spectra internal nuclear structure suggest that there was a structure inside the Yukawa model discovery of atom. Similarly the discrete kinetic energies in an muon, then meson even alpha particle energy spectrum suggest a more particles. structure in the nucleus. click Quarks et al: Explains observed particles click Again the discrete kinetic energies in the fragments of proton - neutron collisions suggested a structure inside these particles, hence the quark model. 35 Chart of how the types of particles relate click 36 Chart of quarks and leptons click 37 Mesons: made of one quark and one antiquark, positive, negative or neutral, examples: Pion, K-meson, over 100 click Baryons: made of three quarks, +2 to -2 in charge, examples: neutron, proton, Lambda, Sigma, and …, about 100, … click double charmed bottom, etc click 38 click How quantitative do you go? click Which units? MeV, Joules click Fusion Reaction: 2D + 2D = 4He click Calculation steps: 1. Mass of 2D, 2. Mass of 4He, 3. Mass diff, 4. Energy release click 39 Production of light electromagnetic wave by accelerating charges, synchrotron radiation at a tangent to a circle, light from transitions between energy levels. These topics don’t seem to link to the rest of Unit 1. So, how do you approach these aspects? click Quarks and leptons are fundamental. Leptons include electrons and neutrinos. Mesons and Baryons have different numbers of quarks. Protons and neutrons are baryons. Note their names. In order of increasing mass left to right and charge values up and down. Only basic information is presented. Note: The Tau particle has a mass equal to that of a Gold atom! Note the range of their charge. Because there are so many particles, it is hard to come up with new names, hence .... Students can use the quark names to come up with one themselves. This graph is a common feature of current text books, but the inclusions of E = mc2 in the study design makes the task quantitative. Also look at the label and units on the Y-axis. So ... Which units do you use? probably both. Consider an example: How would you do this? Look up the mass of Deuterium and double it, and then Helium 4, Subtract Multiply the answer by c2 The difficulty is that to obtain a realistic answer, the initial mass values need to have six digits. Care is needed in how these are approached with Year 11 students. It may be advisable not to spend much time of these. 40 41 Cosmology So much descriptive content … How do you approach it? click Brian Cox video click Images and graphs click Story line click Diagram of the expanding universe click 42 Graph of the temperature of the universe against Time. Big Bang Model: Expanding, intensely hot gas of elementary particles. Explains observable universe back to 1 second. click 43 Graph of the size of the universe against Time. click Big Bang model: Explains Hubble constant, background radiation, click proportion of H, He and Li. click, click Does not explain i) uniformity of universe, ii) universe before 1 sec and iii) energy density of the universe, click but inflationary model does. click 44 45 Practical Activities Radioactivity Pracs Dice pracs click Assessment Tasks an annotated folio of practical activities data analysis design, building, testing and evaluation of a device an explanation of the operation of a device a proposed solution to a scientific or technological problem a modelling activity a media response a summary report of selected practical investigations a reflective learning journal/blog related to selected activities or in response to an issue a test comprising multiple choice and/or short answer and/or extended response click Images and graphs can be effective as well. Lots to explore and digest in this image. The wineglass effect at the bottom is the Inflation Effect. This is a graph of the temperature of the universe against Time. The long straight line is the expanding universe, cooling as it goes, according to the big bang theory. The blip in the shaded section is the Inflation model. There are a few too many lines here, but the straight lines angling down show the expanding universe according to the big bang theory. It explains some things but not others. The inflation model suggests the universe increased by a factor of 1050 in about 10-20 second. Not many These are all the possible assessment tasks in the study design. There are several new ones, se italics on the left. But most are prac related, so not many apply to this Area of Study. 46 47 48 49 50 What’s left? a media response Evaluation of responses in an online discussion click a reflective learning journal/blog related to selected activities or in response to an issue a test comprising multiple choice and/or short answer and/or extended response click Unit 2: Motion Motion Area of Study (with extra content) Options ( 12 on offer) Practical Investigation ( on any of above) click Q’ns: 1. Do you split Motion or not? click 2. Managing several options at same time? click Options: Why you should run more than one option. click The number of options that students do over the two years has dropped from 4 to 3 and now to 1. click Enrolment data indicates that since the introduction of Detailed Studies, the proportion of Year 11 Physics students staying on to do Year 12 physics has steadily increased, both boys and girls. Conclusion: Students value them. click Students learn about options as well as their own. click Motion: Largely the same, but with extra content: Torque click Rotational equilibrium click Differences: click Description of Force: Force on A by B click Word ‘Weight’ is not used in the study design. click How do you approach these changes? click Unit 2 Options Suggestions for managing more than one option: click Student learning: individual, team based or jigsaw method, not teacher directed. click Resources are prepared for several options. click Each teacher decides which ones they are comfortable offering. click Allow students to choose, with a minimum number of students ( eg 4) required for an option to proceed. click Teacher’s role: monitor, guide, support. click Reporting back to the whole class. click Not many choices left. An evaluation of responses in an online discussion may be worth considering. With extra content, Motion may take up to 8 weeks, but it may be unproductive to put options or PI in between. Also many teachers have already offered four of the 12 as Detailed Studies, so the expertise and resources is already there. Practical activities and assessment tasks will probably be unchanged. Are these changes significant? More independent learning Requires preliminary work to set up Allows for team to share the workload Has potential to be a very rewarding experience. 51 Options: Assessment Need to be consistent, but not onerous. Some possibilities are: click an annotated folio of practical activities a media response a summary report of selected practical investigations a reflective learning journal/blog related to selected activities or in response to an issue 52 Practical Investigation Now a separate Area of Study. The topic a student investigates can come from Motion or any of the 12 Options. Now more substantial, the student ‘designs and undertakes an investigation involving two independent variables one of which should be a continuous variable. A logbook must be maintained ….’ Topics can include ‘construction and evaluation of a device’. 53 Requires class time for planning, design, implementation, data analysis and writing up click Plenty of topics for students to choose from. click Preferable to report as: click – A log book with click – A summary in the form of an electronic poster, (e.g. a single powerpoint slide, templates are available). click 54 Year 11 Exam Consider: assessing the whole year, in preparation for the Year 12 exam, Including generic questions on the practical investigation. Students assessing presentations could also be included. A more significant task with more class time. Changes that impact on your planning. Study design suggests 7 - 10 hours, about 3 weeks Check vicphysics.org on EPI's A daily record of plans, data, graphs, ideas for final report. Very much a summary, just the main highlights, the log book is the substance. The Year 12 exam will have questions on the EPI, so prepare the students in Year 11, see link above for examples.