Exam Revision Multiple Choice
advertisement
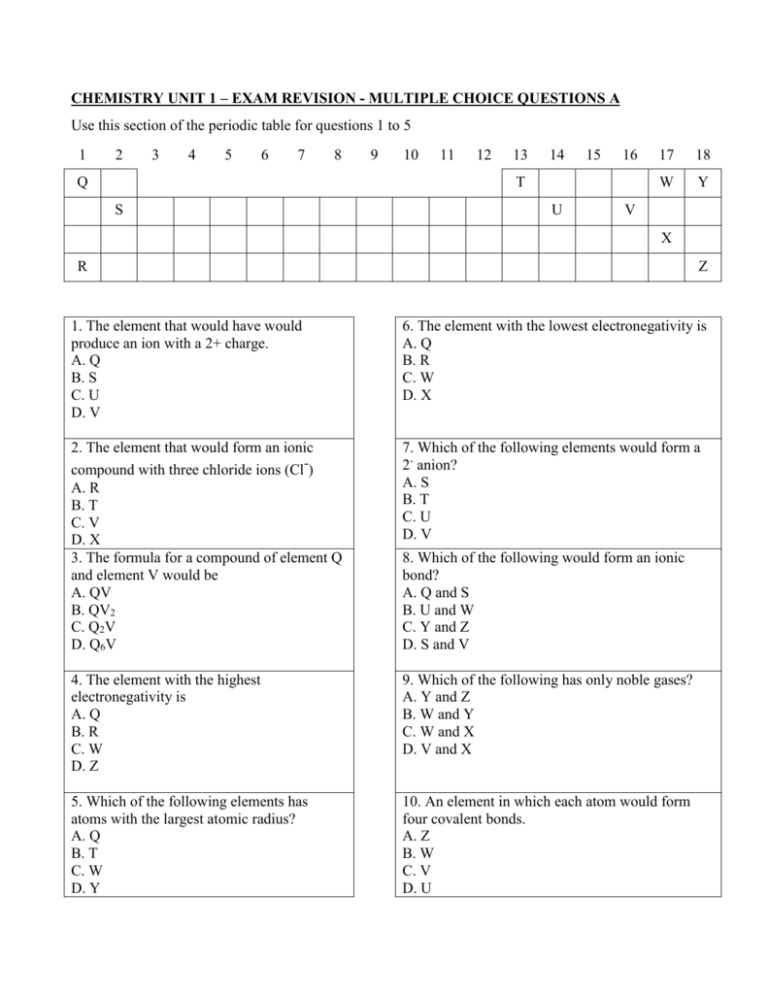
CHEMISTRY UNIT 1 – EXAM REVISION - MULTIPLE CHOICE QUESTIONS A Use this section of the periodic table for questions 1 to 5 1 2 3 4 5 6 7 8 Q 9 10 11 12 13 14 15 16 T S U 17 18 W Y V X R Z 1. The element that would have would produce an ion with a 2+ charge. A. Q B. S C. U D. V 6. The element with the lowest electronegativity is A. Q B. R C. W D. X 2. The element that would form an ionic compound with three chloride ions (Cl-) A. R B. T C. V D. X 3. The formula for a compound of element Q and element V would be A. QV B. QV2 C. Q2V D. Q6V 7. Which of the following elements would form a 2- anion? A. S B. T C. U D. V 4. The element with the highest electronegativity is A. Q B. R C. W D. Z 9. Which of the following has only noble gases? A. Y and Z B. W and Y C. W and X D. V and X 5. Which of the following elements has atoms with the largest atomic radius? A. Q B. T C. W D. Y 10. An element in which each atom would form four covalent bonds. A. Z B. W C. V D. U 8. Which of the following would form an ionic bond? A. Q and S B. U and W C. Y and Z D. S and V CHEMISTRY UNIT 1 – EXAM REVISION - MULTIPLE CHOICE QUESTIONS B 1. Inside the nucleus of a 115B atom are: A. 5 protons and 6 neutrons B. 5 neutrons and 6 protons C. 5 protons and 5 electrons D. 5 protons and 11 neutrons 2. The main source of hydrocarbons is A. food B. plastics C. petroleum D. the atmosphere 3. The formula for octene is A. C8H8 B. C8H14 C. C8H16 D. C8H18 4. Which of the following is NOT an alloy? A. cupro-nickel B. iron C. solder D. brass . 5. Different forms of the same element are called A. isotopes B. isomers C. alloys D. allotropes 6. A 9.20g sample of calcium oxide (CaO) has 6.58g of calcium. The percentage composition of oxygen in calcium oxide is: A. 28.5% B. 39.4% C. 50.0% D. 71.5% 7. Going across a Period in the Periodic Table the A. metallic character increases and the atomic radius increases B. metallic character increases and the atomic radius decreases C. metallic character decreases and the atomic radius increases D. metallic character decreases and the atomic radius decreases 8. In a flame test the characteristic colour is produced by A. the anion as the electrons move to a higher energy level B. the cation as the electrons move to a higher energy level C. the anion as the electrons move to a lower energy level D. the cation as the electrons move to a lower energy level CHEMISTRY UNIT 1 – EXAM REVISION - MULTIPLE CHOICE QUESTIONS C 1. When potassium K reacts with water H2O the gas produced is: A. potassium B. oxygen C. hydrogen D. carbon dioxide 2. A compound has the empirical formula CH. If one mole of the compound has a mass of 52g then its molecular formula is: A. C8H8 B. C6H6 C. C4H4 D. C2H2 3. When metal is heated to red hot and is allowed to cool slowly it will be: A. softer and more brittle B. softer and less brittle C. harder and more brittle D. harder and less brittle 4. Which of the following is saturated? A. C7H7 B. C7H12 C. C7H14 D. C7H16 5. The alloy made of tin and lead is: A. cupro-nickel B. stainless steel C. solder D. bronze 6. What must a monomer have to create a polymer by addition polymerization? A. a dipole B. a crosslink C. a hydrogen bond D. a double carbon bond 7. Isomers have: A. the same molecular formula, but a different structural formula B. the same elements, but in different numbers C. the same general formula, but differ by one –CH2 D. only one element arranged in a different structure CHEMISTRY UNIT 1 – EXAM REVISION - MULTIPLE CHOICE QUESTIONS D 1. The ground state electron configuration for 31Ga is: A. 1s2, 2s2, 2p6, 3s2, 3p6, 3d6, 4s2, 4p3, 5s2 B. 1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s2, 4p1 C. 1s2, 2s2, 2p6, 3s2, 3p2, 3d5, 4s2, 4p6, 4d4 D. 1s2, 2s2, 2p6, 3s2, 3p6, 3d9, 4s2, 4p2 2. Who in 1911 showed that molecules are mostly made up of empty space with a tiny nucleus, by firing alpha particles at gold foil? A. Ernest Rutherford B. Niels Bohr C. John Dalton D. Dmitri Mendeleev 3. A methyl group has the formula A. –CH B. –CH2 C. –CH3 D. –CH2CH3 4. Which of the following molecules is non-polar? A. H2O B. OCl2 C. NH3 D. N2 7. Which of the following numbers definitely has three significant figures? A. 4000 B. 40.0 C. 0.004 D. 400 8. In a flame test the characteristic colour is produced by A. the anion as the electrons move to a higher energy level B. the cation as the electrons move to a higher energy level C. the anion as the electrons move to a lower energy level D. the cation as the electrons move to a lower energy level