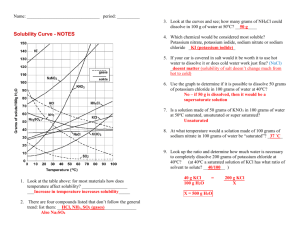
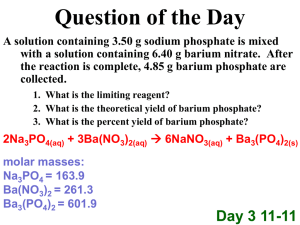
Set #1 1. How many significant figures in: 35.080 0.00760 2. What is
advertisement

Set #1 1. How many significant figures in: 35.080 0.00760 2. What is the SI unit for: mass temperature quantity of a substance 3. Convert the following: 300mg = ____g 7000 km = ____ Gm 275,000 μs = ______ s Set #3 1. What are the 3 parts of an atom? 2. What are their charges and locations? 3. What was found out through the gold Foil experiment? Whose experiment. was it? 4. What is the atomic number, mass number and atomi mass? 4. How would you find the density of an irregular solid? 5. What is the density of a 13g piece of metal with a volume of 12.5 cm3? Set #2 1. Identify the following as heterogeneous or homogeneous mixtures: soil kool-aid brass milk 2. Identify the following as chemical or physical changes: tearing paper melting ice burning coal boiling water forming a precipitate 3. Identify the following as chemical or physical properties: boiling point reactivity with acid density mass reactivity in air flammability 5. What is an isotope? How many protons, neutrons and electrons are in: Calcium – 42 Gold – 198 48 4+ Ti Set #4 1. How many protons, electrons and neutrons are in Phosphorus-32? 2. What is the average atomic mass for an element with these isotopes? 19.8% 10.013amu 80.2% 11.009 amu 3. What is the atom above? 4. What is an exothermic reaction? 4. What is the difference between atomic mass and mass number? 5. Identify the following as elements, compounds or mixtures: salt sand silver water Stainless Steel clay 5. Who discovered the electron? What experiment is associated with this? Set #5 Set #7 1. How do ions get a + or – charge? How do we determine the exact charge for: C K I He Sr 2. What is the charge of the following: F Mg Al Ne Zn Pb 3. How do elements’ electron clouds become stable? (tell 3 conditions) 4. Define groups and periods. 1. What are the prefixes for: 4 6 10 2. What is the formula for: Disulfur pentoxide triarsenic heptabromide Carbon Monoxide 3. What is the formula for: Lead (IV) Bromate Cuprous hydroxide Stannous Phosphate 4. What is the formula for: Nitrous Acid Sulfuric Acid Hydrobromic Acid 5. What is the group name for these elements: Kr Ba Li Ag Br O Set #8 The Mole Set #6 1. Name the following: NaCl K3PO4 Sn(ClO)4 Ag(IO2) · 4H2O 2. Name the following: SI2 N3O2 PCl5 1. a. Determine the molar mass of Potassium Phosphate b. Given 125 grams of Potassium Phosphate, how many moles? c. If 1.75 moles of Potassium Phosphate are needed, how many grams is that? 3. Name the following acids: HI H2SO3 H3PO4 2. How many atoms are in 59.0 grams of Sodium? 4. What is the formula for: calcium hydroxide Ferric Nitrate Vanadium(V) Acetate Lithium perbromate hexahydrate 3. Find the percent composition for Gold(III) Phosphate. 4. Determine the Molecular Formula for: C = 92.3 g H = 7.70 g And Molar Mass = 78.0 g/mol Set #9 1. Draw the Lewis structure for the following: HBr H2O C2H6 BH3 PCl5 2. Give the shapes of the molecules above 3. Tell if the bonds and the molecules are polar or nonpolar 4. Give the intermolecular forces for the above 5. A density lab determines the density of Copper is 8.05 g/mL. A reference book shows the True density as 8.95 g/mL What is the % error? 6. How many sig figs? a. 0.0025 s b. 175,000 m c. 6.500 g Set #10 Nuclear 1. What are the symbols for alpha, and beta particles? 2. Neptunium-237 undergoes alpha decay. Write the eq. 3. Phosphorus-28 undergoes beta+ decay. Write the eq. 4. Complete the eq’ns: a. 239 94 b. 14 7 Pu+ 24 He 01 n+____ N + _____ 14 6 C + 11 H 5. Given 500. grams of radioactive Strontium-90. How many grams will still be radioactive after 5 half-lives?