Hess's Law Lab
advertisement

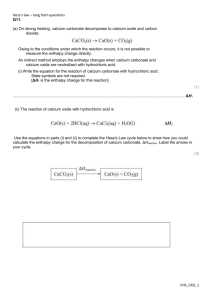
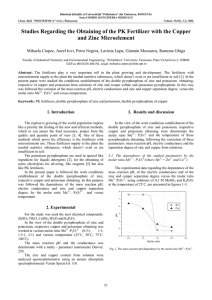
Hess’s Law Lab Purpose The purpose of this lab is to use Hess’s Law to determine the molar enthalpy of combustion of zinc using calorimetry. Introduction Hess’s Law enables us to determine the molar enthalpy of the following reaction (1) Zn(s) + ½ O2(g) → ZnO(s) using the series of reactions listed below. (2) Zinc reacts in acid to form hydrogen and a salt: Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g) ΔH2 = ? (3) Zinc oxide reacts with acid to produce water and a salt: ZnO(s) + 2 HCl(aq) → ZnCl2(aq) + H2O(l) ΔH3 = ? (4) The formation of liquid water from its elements H2(g) + ½ O2(g) → H2O(l) ΔH4 = ? Procedure Safety precautions: Hydrochloric acid is very corrosive. Suitable protective gear should be used. All spills should be cleaned up quickly and any skin that has come into contact with acid should be immediately and thoroughly rinsed with cold water. 1. Measure 100.0 mL of 1.00 mol/L hydrochloric acid into a polystyrene cup. Measure the initial temperature of the acid solution to the nearest 0.2 ºC. 2. Measure 1.5 g (to ± 0.01 g) of zinc metal. Add the solid to the solution, stir it, and record the maximum temperature that the solution attains. 3. Dispose of the products as directed by your teacher, and rinse and dry the equipment. 4. Repeat the first three steps, using approximately 2 g of magnesium oxide measured to ± 0.01 g. Analysis *report all final answers in the correct number of significant digits* 1. Calculate the molar enthalpy change for reaction (2) and (3) [6 pts] 2. Determine the standard molar enthalpy of formation of water [1 pt] 3. Using Hess’s Law, determine the molar enthalpy of combustion of zinc [8 pts] 4. The accepted value for the molar enthalpy of combustion of zinc is -350.5 kJ/mol. What is your percent accuracy? What is your percent error? Percent error= ± experimental value – theoretical value x 100% [4 pts] theoretical value 5. What measured values limited the precision of your ΔH value? How? [4 pts]