Review worksheet
advertisement

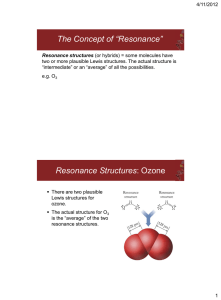
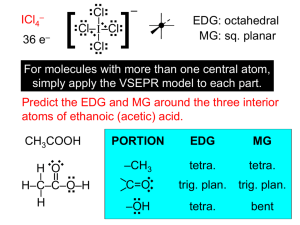
Chemistry. Review of Bonding. Name 1. Write Lewis dot structures for the following elements P Ca Ti Kr Sn Au Br 2. Name the following compounds Ag2S Cu3(PO4)2 NaHSO4 IF7 PCl3 N2O5 NO Ca(ClO)2 HNO3(aq) NO2 NiSO4 H3PO4(aq) N2O Fe2O3 S2Cl2 N2O4 P4S3 SnCl4 ClF3 (NH4)2CO3 Mg(OH)2 H2S Co(ClO2)3 Co(ClO2)2 3. Explain what resonance means. Using only one of these (COCl2, O3, CHO2-1, POCl3) show how resonance is represented in Lewis structures. 4. Calculate the oxidation state of the italicized elements in the following compounds Ho2O3 Nb(BrO)3 H4P2O6 NaClO2 Zn3(PO4)2 5. What is the difference between a covalent bond and coordinate covalent bond? 6. How do you know that the molecule you are looking at is polar or nonpolar? 7. Explain how bond strength (bond dissociation energy) & bond length are associated with the type of bond (single, double & triple). 8. Complete the following table AROUND CENTRAL ATOM Molecule or Ion Cl2O NF3 CS2 SiF5-1 SO3 NH2OH XeF2 BrF4-1 XeF4 BrF5 C2H2Cl2 C2H2Cl2 different than one above Lewis Dot # of bonding regions # of lone pairs VSEPR formula shape hybridization Geometric Formula (label pi and sigma bonds and value of the angles) Polar or Nonpolar?
















![[1] for the structure](http://s2.studylib.net/store/data/009991918_1-96801ab7d3a6d934a397fe244b8cf290-300x300.png)