Review Notes 1
advertisement

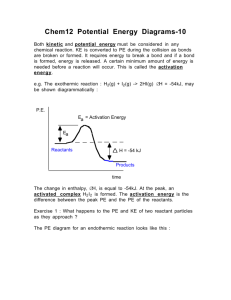
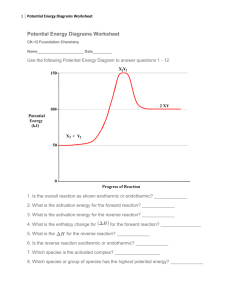
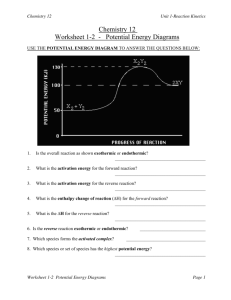
Chemistry 12. Unit 1 Review A: Reaction Kinetics (Introduction) 1. Give examples of reactions proceeding at different rates. 2. Describe rate in terms of some quantity (produced or consumed) per unit of time. 3. Experimentally determine rate of a reaction. Calculate rate of reaction from a given graph (slope w/ units). 4. Identify properties that could be monitored in order to determine a reaction rate. 5. Recognize some of the factors that control reaction rates. 6. Compare and contrast factors affecting the rates of both homogeneous and heterogeneous reactions. 7. Discuss situations in which the rate of reaction must be controlled. 8. Predict if a reaction rate is spontaneous or not in terms of enthalpy and entropy. B: Reaction Kinetics (Collision Theory) 1. Know the following: - reactions are the result of collisions between reactant particles. - all collisions are successful when there is sufficient kinetic energy (KE) and collide with favourable geometry. - to increase the rate of a reaction one must increase the frequency of successful collision. Understand the KE vs. Particle distribution graph. - energy changes are involved in reactions as bonds are broken and formed (exothermic and endothermic considerations). 2. Describe the activated complex in terms of its potential energy (PE), stability, and structure. 3. Define activation energy. 4. Describe the relationship between activation energy and rate of reaction. 5. Describe the changes in KE and PE as reactant molecules approach each other. 6. Draw and label PE diagrams for both exothermic and endothermic reactions, including, activation energy, and the energy of the activated complex. Know how to determine the activation energy and enthalpy for both forward and reverse reaction 7. Relate the sign of H to whether the reaction is exothermic or endothermic 8. Write a chemical equation including the energy term (given a value) and vice versa. 9. Describe the role of the following factors in reaction rate (use collision theory): - nature of reactants - concentration - temperature - surface area C: Reaction Kinetics (Reaction Mechanisms and Catalysts) 1. Use examples to demonstrate that most reactions involve more than one step. Understand why some reactions occur with more than one step. 2. Describe a reaction mechanism as the series of steps (collisions) that result in the overall reaction. Each step is known as an elementary step. Provide examples of several reaction mechanisms. 3. Define catalyst. 4. Compare and contrast the PE diagrams for a catalyzed and uncatalyzed reaction in terms of reaction mechanism and activation energy. 5. Identify reactant, product, reaction intermediate, and catalyst from a given reaction mechanism. 6. Describe the uses of specific catalysts in a variety of situations. A. Reaction Kinetics 1. Consider the following reaction: Na2CO3(s) + 2 HCl(aq) ------ > 2NaCl(aq) + H2O(l) + CO2(g) A 45.0g sample of sodium carbonate is placed in a flask and HCl is added. The reaction consumes 2.15 g of sodium carbonate in 1.0 minute. a) Calculate the average rate of reaction in moles per second. b) How many moles of sodium carbonate is left after 6.0 minutes? 2. One mechanism for the destruction of ozone in the upper atmosphere is O3(g) + NO(g) --------- > NO2(g) + O2(g) NO2(g) + _____ -------- > NO(g) + ______ fast --------------------------------------------------------Overall: O3(g) + O(g) slow ------- > 2O2(g) a) What are the missing substancess? b) Which specie is an intermediate? Define the term intermediate. c) Which specie is a catalyst? Define the term catalyst. d) Make a rough sketch of the potential energy diagram for the reaction. Assume this is an exothermic reaction. e) Define “rate determining step”. For the reaction above, which one is the rate determining step? f) Define reaction mechanism: 3. Air is mainly a mixture of nitrogen and oxygen molecules. These molecules can react to produce nitrogen dioxide, a red-brown gas. N2(g) + 2O2(g) ------ > 2NO2(g) Even though there are more than 4 billion collisions per second between Nitrogen and oxygen, the amount of nitrogen dioxide present after a year is too small to be detected. a) Using collision theory, why is this reaction slow? b) Describe how the rate of reaction can be increased.