Chapter 6 Homework
advertisement
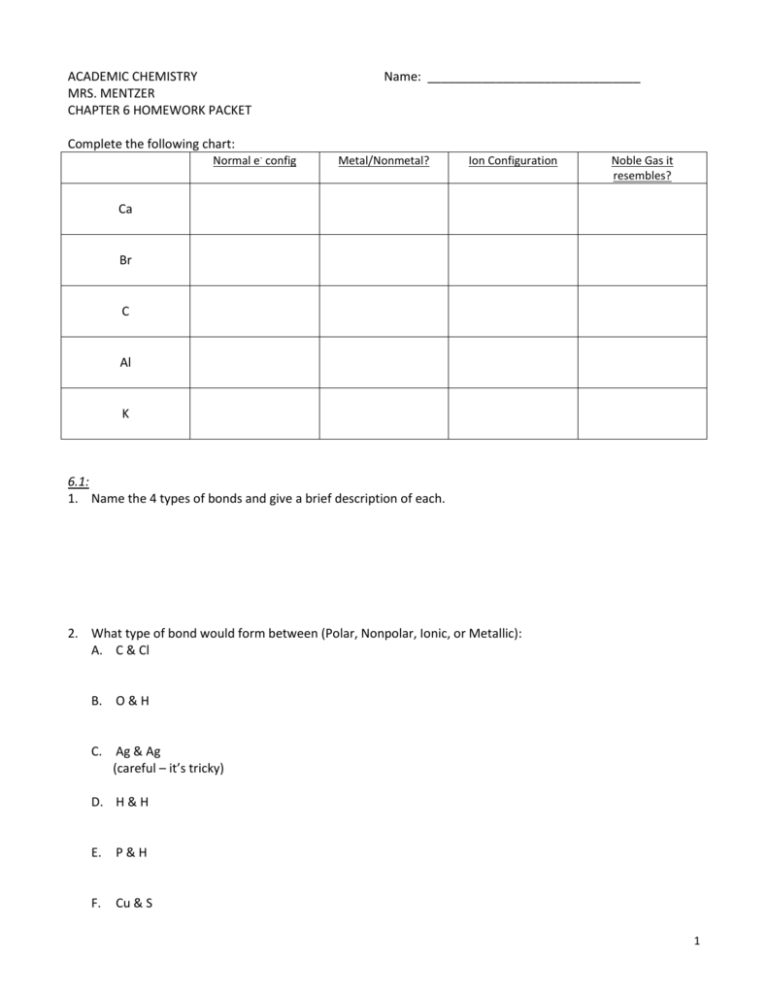
ACADEMIC CHEMISTRY MRS. MENTZER CHAPTER 6 HOMEWORK PACKET Name: _______________________________ Complete the following chart: Normal e- config Metal/Nonmetal? Ion Configuration Noble Gas it resembles? Ca Br C Al K 6.1: 1. Name the 4 types of bonds and give a brief description of each. 2. What type of bond would form between (Polar, Nonpolar, Ionic, or Metallic): A. C & Cl B. O & H C. Ag & Ag (careful – it’s tricky) D. H & H E. P&H F. Cu & S 1 G. Cl & F H. N & H I. S&O J. N&O 6.2: 1. Draw Lewis Structures for: (Hint – these are all single-bonded molecules!) A. CH3I B. NH3 C. H2S D. C3H8 E. H2O F. NH2Cl 2 2. Draw Lewis Structures for: (Hint – these are all multiple bonded molecules!) A. CO2 B. C2H2 C. C2HCl D. HCN 3. Define bond length 4. Define bond energy 5. How many total electrons in a: A. single bond B. double bond C. triple bond 6. How many electron pairs in a: A. single bond B. double bond C. triple bond 3 6.3: 1. Draw Lewis Dot diagrams (add as many of each as you need) for: A. K + Cl B. Li + O C. Rb + F D. Mg + Cl E. Sr + O 2. List the similarities and differences between ionic and molecular compounds. 6.4: 1. Explain the steps involved in forming metallic bonds. 4 6.5: 1. Determine the shape of: A. NH3 B. CO2 C. CBr4 D. HCN E. SF2 2. Determine if molecule is dipole, NPWPS, or nonpolar. (Hint – you already did all the work in #1!) A. NH3 B. CO2 C. CBr4 D. HCN E. SF2 5








