Worksheet on Bohr's model (and light and waves and
advertisement

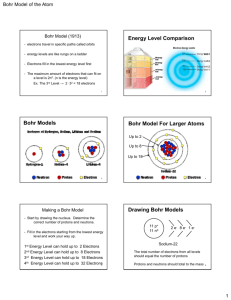
Worksheet on Bohr’s model (and light and waves and electromagnetic radiation) Name ________________________________ Drawing the Bohr atom is very similar to drawing the Rutherford model of the atom… 1. Determine the number of protons, neutrons, and electrons in the atom 2. Draw the correct number of protons and neutrons in the nucleus (be sure to mix them up within the nucleus) 3. Place up to two electrons in the first energy level (never more) 4. Place up to eight electrons in the second energy level (never more) 5. Continue this with electrons until you have placed all the electrons in the energy levels (up to 18 electrons in the third shell, 32 electrons in the fourth level, and 50 electrons in the fifth level) Examples…draw Neils Bohr’s model of the Oxygen – 18 atom… 1. The mass number is 18 and the atomic number 8, so the atoms has 8 protons, 10 neutrons, and 8 electrons… 2. Build the nucleus with neutrons and protons 3. For eight electrons, two will go into the first level, and the other six will go into the second energy level… 4. Now check to make sure that you have enough of the three subatomic particles… Now, things for you to do… 1. Draw the Bohr model for this neutral atom…Boron – 9 2. Draw the Bohr model for the Fluorine ion with a mass of 19 and a charge of minus 1… 3. Draw the Bohr model for the neutral Chlorine – 35 atom… 4. Answer the following questions about the electromagnetic spectrum… a) Which of these has a greater amount of energy in the waves – gamma rays or ultraviolet waves? b) Which has a higher frequency – red or blue light? c) Which has the shorter wavelength – green or yellow light? 5. How does a gas give off energy when it is put into a glass tube and has electricity run through it? Answer in a complete sentence or two… 6. Label all the parts of this wave…you should have six terms labeled…