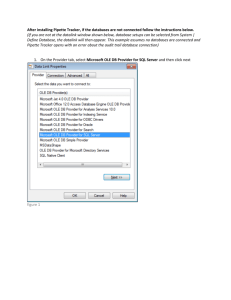
Charles Law Lab
advertisement

Charles Law Lab Purpose: To investigate Charles Law Materials: two large beakers, hot plate, ice, "pulled" and cut beral pipette, tongs, thermometer Procedure: 1. Fill the entire beral pipette with water and count the number of drops (this will be your V1) the pipette contains while heating a beaker 3/4 full of water on a hot plate. 2. Taking care not to let the hot water go above 80 degrees (the pipette plastic will warp otherwise - you may want to remove the beaker from the hot plate before you do step three) hold the pipette gently but firmly by the pulled stem with your tongs. Take care that the open tip is pointing down when you submerge the pipette completely in the hot water. Bubbles will begin to come out of opening - when the bubbles stop or slow considerably proceed to the next step. (Make sure you record the temperature of the hot water - this will be your T1 - don’t forget to add 273.15 K!) 3. Very quickly transfer the pipette from the hot water to the cold water - making absolutely certain that the open tip of the pipette goes in the cold water first (NOT the bulb). Keep submerged for about 30 seconds. The cold water temperature will be your T2 (plus the 273.15 of course). Water will be pulled into the pipette - to get V2 you will subtract the number of drops pulled into the pipette from V1. Data: Number of drops in entire pipette: _____________ Number of drops sucked in : _____________ Temperature of hot water : _____________ Temperature of cold water : _____________ Calculations: See procedure for what numbers to plug into Charles Law and calculate to see if the two sides of the equation are equal to each other. Make sure you explain possible sources of error. Explain how the results in this lab verify Charles Law.