Hydrated Crystal Lab Directions
advertisement

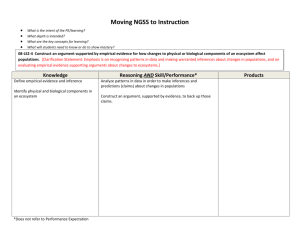
Hydrated Crystal Lab Directions 1. Read pages 213 – 215 of your textbook. 2. Working with a partner, determine an experimental method that will allow you to verify the empirical formula for Epson salt. The maximum amount that you may use is 5.0 grams of Epson salt. 3. After you develop a written procedure, show it to the teacher before proceeding. 4. Create a data table similar to the one at the bottom of this page 5. In your experiment, you must solve for the following: The experimental empirical formula for the compound The percent error in you experiment (the true formula is magnesium sulfate hepta hydrate) Repeat the experiment and make at least one change that you think will reduce the percent error. 6. In your conclusion, be sure to discuss: What caused the error Did your change reduce the error, why or why not? What else could you have tried to help reduce the error? Sample data table: Trial # and change made 1 – no changes 2– changed … Mass of crucible Initial mass Final mass Mass of water Mass of MgSO4 Empirical formula Percent error