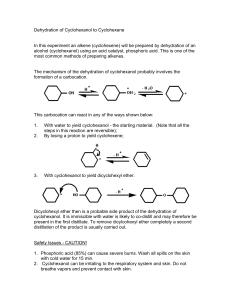
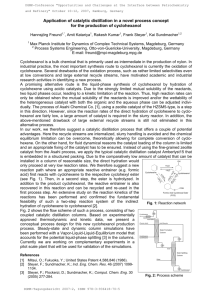
CHM412 EXPERIMENT 8-fazni
advertisement
Page 1 of 3 CHM 412 EXPERIMENT 8 PREPARATION OF CYCLOHEXENE FROM CYCLOHEXANOL Objective Synthesis of cyclohexene from cyclohexanol via water elimination with acid catalysed. Pre lab assignment 1) Find the density for cyclohexanol and cylohexene. State the reference(s). (3 marks) 2) Show the calculation for the mass and moles for 20 ml of cyclohexanol. (4 marks) Prerequisite 1) Techniques as in experiment 1 will be required in experiment 8. Theory One of the common method to produce alkene is through elimination. There are two elimination reactions learned by students at this level for production of alkenes. Alkenes can be made from water elimination of alcohol with acid catalysed and dehydrohalogenation of alkyl halides in alkaline in alcoholic condition. In water elimination of alcohol, strong acid is required for the protonation of the molecule. Carbonium intermediate formed after the elimination of water molecule, followed by lost of proton with the formation of alkene as shown in the equation below. H OH + + H OH2 + H + H2O H + H2 O Page 2 of 3 Apparatus Thermometer -1 still head -1 condenser - 1 round bottom flask (50ml) - 1 receiving flask (50 ml) -1 rubber hoses - 2 conical flasks (150-250 ml) -2 adapters – depending on the set available retort stands - 2 pc gravity funnel -1 water bath separatory funnel and stopperv(150ml) -1 set test tube - 1 Chemicals Cyclohexanol 85% phosphoric acid sodium chloride solid 10% sodium carbonate solution anhydrous magnesium sulfate boiling chips ice cubes. 1-2% KMnO4 solution Procedure 1. 2. 3. 4. 5. 6. 7. 8. Set up a simple distillation apparatus. Add around 10-11.5ml of 85% phosphoric acid in a round bottom flask. Add in 20 ml of cyclohexanol and a few boiling chips. Distill the reaction mixture. Immerse the receiving flask up to its neck in an ice bath to avoid loss of any product (b.p. 83C). Heat the distillation flask carefully so that a steady distillation rate is obtained. Do not allow the temperature of the distilling vapors to exceed 103C. Stop heating the reaction mixture when only a few ml of liquid remain in the distilling flask or when the vapors exceed 103C. Saturate the distillate by adding solid sodium chloride portion wise with swirling (Note: stop adding the salt when it is not dissolve anymore). Try to keep the excess sodium chloride to a minimum. Page 3 of 3 9. 10. 11. 12. 13. 14. 15. Now, neutralize any acid by adding just enough 10% sodium carbonate (Na2CO3(aq)) solution to the mixture with swirling to make the aqueous layer basic (use blue litmus paper to the solution). Note: every a few drops of Na2CO3(aq) added, test the solution by putting a drop of the solution on a blue litmus paper until it the blue litmus paper remained blue. Transfer the mixture to a separatory funnel and drain of the aqueous layer. At this stage you need to determine yourself which layer is aqueous using the technique you learned in experiment 1. Pour the organic layer into an Erlenmeyer/conical flask and dry it with just enough anhydrous magnesium sulfate. Filter the product into a clean, dry, round bottom flask via gravity filtration. Redistill the dried cyclohexene. Record the boiling point and measure the volume of the product. Test the product form with a few drops of KMnO4(aq), observe the colour change. Questions: 1) Find the moles of cylohexene formed in the experiment. (2 marks) 2) Calculate the expected mass of pure product cyclohexene using the number of moles cylohexanol used in the experiment based on the equation. (2 marks) 2) Show the calculation for the percentage yield/conversion of cyclohexanol to cyclohexene. (2 marks) 3) Suggestion another simple chemical test for the product instead of KMnO4(aq) and state the observation. (2 marks) 4) Draw the products that would be obtained by the dehydration of the following molecules and state which are minor and major products. (4 marks) (a) 2-heptanol (b) 2-methyl-1-cyclohexanol 5) Why the amount of 85% phosphoric acid is not necessarily to be exact amount as cyclohexanol? (1 mark)