Anactid Titration
advertisement

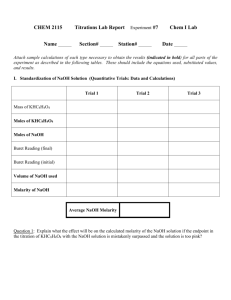
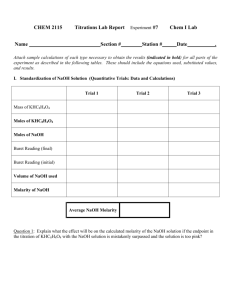
Neutralizing Power of Antacids Data, Calculations, and Results (Note: This template should be completed electronically and inserted into your formal lab report for this experiment. Alone, it does NOT comprise your entire report, and you should be able to create one complete document with content prior to and after this section. Also, make every effort to format tables and equations so that they are not split between pages. If you must split a table, please use a note to indicate that it is continued on a subsequent page. You should delete this note as part of your editing. ) Part I Data: Molarity of NaOH used Part II Data: TRIAL VOLUME OF HCl TITRATED INITIAL BURET READING (mL OF M NaOH) FINAL BURET READING (mL OF M NaOH) 1 2 3 Part II Calculations: EQUATION TRIAL 1 EXAMPLE Moles of NaOH used in titration of 25.00 mL of ‘stomach acid’ Molarity of stomach acid Average Molarity of stomach acid Part II Results: TRIAL 1 TRIAL 2 TRIAL 3 mL of NaOH(aq) added Moles of NaOH Molarity of “stomach acid” Average Molarity of stomach acid Part III Data Brand of Antacid used Amount of Antacid used Recommended dose of Antacid # of doses per package Price of Antacid Total Volume of HCl added Part III Calculations EQUATION EXAMPLE Total number of moles of acid added to the antacid Part III Results Total volume of acid added to antacid Total number of moles of acid added to the antacid Part IV Data TRIAL 1 mL OF ANTACID SOLUTION TITRATED INITIAL BURET READING (mL OF M NaOH) FINAL BURET READING (mL OF M NaOH) 2 Part IV Calculations EQUATION Moles of acid in excess in each titration (per pipette) Excess molarity of acid Total number of moles of acid in excess Total number of moles of acid neutralized by one tablet or teaspoon of antacid Average moles of acid neutralized by one tablet or teaspoon of antacid Moles of acid neutralized per dose Doses of antacid per dollar cost of antacid EXAMPLE Moles of acid neutralized per $1.00 Part IV Results TRIAL 1 TRIAL 2 Moles of acid in Excess Excess molarity of HCl Total number of moles of acid in excess Total moles of acid neutralized by one tablet or teaspoon of antacid Average number of moles of acid neutralized by one tablet or teaspoon of antacid Average number of moles of acid neutralized per dose of antacid Doses of antacid per dollar Moles of acid neutralized per $1.00 Antacid Information Antacid Rolaids Tums Active Ingredients 550 mg CaCO3 and 110 mg Mg(OH)2 per tablet 500 mg CaCO3 per tablet Price Quantity Dose $4.99 150 tablets 3 tablets $5.79 150 tablets 3 tablets Mylanta Maalox Milk of Magnesia 200 mg Al(OH)3 200 mg Mg(OH)2 per teaspoon 200 mg of Mg(OH)2 200 mg of Al(OH)3 per teaspoon 400 mg of Mg(OH)2 per teaspoon $6.29 12 fluid ounces 3 teaspoons $5.89 12 fluid ounces 3 teaspoons $6.49 12 fluid ounces 2 teaspoons Cost Effectiveness Comparison: (Use class average information here and use package information to calculate theoretical values here. Delete this note and the arrows.) Antacid Rolaids Tums Mylanta Maalox Milk of Magnesia average experimental neutralizing capacity per dollar theoretical moles HCl neutralized per dose theoretical neutralizing Capacity per Dollar percent difference