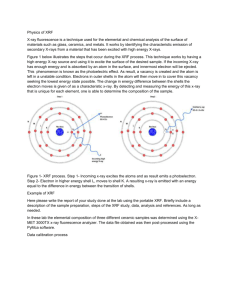
Industrial Applications of Synchrotron Radiation Non
advertisement

Industrial Applications of Synchrotron Radiation Non-destructive testing Characteristics of aircraft and automotive components such as strength and resistance to extreme conditions for welded areas - for example, their response to thermal and mechanical and chemical strain are investigated using synchrotron radiation. X-ray diffraction (XRD) and high-resolution microtomography are used to detect implications of residual stress. Synchrotron radiation can also be used for three-dimensional imaging based in absorption, scattering and phase. Phase microtomography is able to provide 3D information of nucleation and propagation of small fatigue cracks in load bearing components such as wings or skins in aircrafts, rails, car wheels, bearings. For example in cast Al alloys, cracks of length in the 10 μm scale are able to be imaged in high-resolution, with a voxel size of 0.7 μm. (J. Baruchel et al, Scripta Materialia 55 (2006) 41 - 46) These cracks in aircraft aluminium alloys have been observed to arise from atmospheric corrosion which can begin as pitting and intergranular corrosion and can develop into fatigue cracks, stress corrosion cracks or exfoliation. Extensive work has been done using x-ray tomography to monitor the extent of corrosion attack due to different relative humidities and growth of intergranular corrosion under salt droplets on Al alloys. (S. Knight et al, Corrosion Science 53 (2011) 727 - 734) Investigation of porous media is important as they are of interest as lightweight metallic foams or as open structures in solid oxide fuel cells where they determine the gas flow, as well as for use as hydrogen storage in metal organic frameworks. An example of this is the case of syntactic foams used in the oil industry to thermally insulate the pipes transporting hot oil from the extraction fields at a high depth in the cold sea water. The pipes have to prevent the oil from cooling down, and must also resist a 30 MPa hydrostatic pressure. They are composed of a polymer matrix surrounding hollow silica spheres reinforcing the porous structure and preventing it from collapsing under the applied pressure. As these materials are made of air, silica and polymer they are very difficult to observe using standard microscopy techniques, however synchrotron high resolution tomography has been used for this purpose. The determination of the thickness of the hollow spheres (in general in the range 1 – 3 μm) is very important for understanding the physical properties of the material and this was able to be measured to 0.28 μm resolution at ESRF, as shown in Fig. 1. (J. Baruchel et al, Scripta Materialia 55 (2006) 41 - 46) Tomographic slice of a polymer foam containing hollow silica spheres using (left) a 0.7 μm voxel size and (right) a 0.28 μm voxel size. Figure 1: Imaging of soft tissues cannot be performed using conventional x-ray transmission radiography, however a relatively new form of radiography known as Phase-contrast imaging has been developed using synchrotron radiation which brings additional information by diffraction and interference effects in the wave-nature properties of photon beams which are induced by traversing media with varying refractive indices. This tecnology has been able to create high resolution x-ray images of soft tissue structures such as insects with structural details visible in the 10 – 100 μm range. Tomographic images of small structures such as mouse kidneys and termite heads are also able to be generated. Further developments are possible including in-vivo and time dependant studies in biological species with applications in dental imaging and mammography. (J. Jakubek et al, Nucl. Inst. Meth. A, 571 (2007) 69 – 72) A study was made on the capabilities of phase-contrast imaging for real breast tumours and it was found that in all cases, phase-contrast images showed a higher quality than those obtained using conventional x-ray absorption imaging, demonstrating that significant benefits in the detection of breast tumours can be obtained by means of this technique. It was also found that changing the beam energy allows the possibility of either reducing the delivered dose while preserving image quality, or of increasing image quality while keeping the delivered dose, and that a new trade-off between energy and dose has to be indentified according to the specific requirements. (A. Olivo et al, Applied Radiation and Isotopes, 67 (2009) 1033 - 1041) The effects of inhalation of various atmospheric particles on the lungs of mice, phase-contrast imaging was used to to image lung injuries caused by inhalation of vehicle exhaust fumes and metallurgic plant emissions in mice. It was found that in vivo studies of living animals can easily be performed and lung injuries caused by air particles from different areas could be distinguished. From the images of excised tissues, not only hemorrhage spots could be seen, but also the changes of detailed structures such as alveoli and blood vessels. The lung inflammation caused by particles collected in a traffic tunnel was the most serious, followed by those from an industrial area and those from a suburban area. The lung injury process caused by such particles is alveolus wall thickening, hemorrages appearing, alveolus structures damaging and finally fibrosis. These results suggest that phase-contrast imaging is a promising technique for diagnosis of pnemonia. (W. Yue et al, Nucl. Inst. Meth. B, 262 (2007) 304 – 312) Phase-contrast was recently used to study the respirator system of insects which has in the past been very difficult to understand due to the immense variation and complexity in insect anatomy and fundamental questions regarding the relative roles of convection and diffusion as a function of body size, phylogeny, development and life history were unanswered. Synchrotron x-ray imaging was Figure 2: High quality phase-contrast x-ray image of an ant worker. The round structures in the ant's abdomen are air bubbles. able to observe in real time the tracheal system of intact, living insects and is a powerful tool for understanding the internal dynamics involved in respiration. It enables the direct visualisation of the insides of living animals with high spatial and temporal resolution and data collection can be relatively quick and simple. An example is shown in Fig. 2. (J. Socha et al, Respiratory Physiology & Neurobiology 173S (2010) S65 - S73) Phase-contrast imaging was applied to material science with high resolution imaging of an aerospace material comprising two thin sheets of aluminium separated by an epoxy film adhesive, with voids in the adhesive of size 100 μm clearly visible. Furthermore images of a 50 μm Ni foil containing air bubbles and thin glass fibres were recorded as well as a 75 μm thick polyimide film with a 40 μm period structure of depth 20 μm. Further objects of interest included a section of a succulent leaf and a feather showing very highly resolved features. (A. Stevenson et al, Nucl. Inst. Meth. A 199 (2003) 427 – 435) Industrial uses of synchrotron imaging in the rapidly growing field of nanotechnology include detection of microcracks, voids and failures in electronics, products of the metallurgy industry, aerospace and automobile components, new products from energy companys, building materials, petroleum and mining equipment. Nano- and micromorphology in functional glasses and glassceramics, buliding materials, plastics, cosmetics, wood and pulp, foods, drugs and tissues are also of interest. X-ray Fluorescence (XRF) X-ray Fluorescence (XRF) using synchrotron radiation can be used to perform trace element analysis to determine concentrations of impurity elements in materials of interest with a wide range of application. Such analysis is important in silicon circuit technology as metallic impurities can lead to leakage current, gate insulator breakdown or poor threshold voltage control, and the semiconductor industry has certain requirements with respect to trace-element concentrations in their components. Measurements have been performed at Hasylab (Beam L) to quantify the concentration of Ni impurities in Si-wafers with a detection limit of 15 fg (1 fg = 10 -15 g) ie 109 atoms cm-2. An example is shown in Fig. 3. This technique has become so valuable that a joint ESRF/industry initiative is funding a specially dedicated experimental station for these investigations. (P. Wobrauschek et al, Spectrochimica Acta Part B 52 (1997) 901 - 906) Figure 3: XRF spectrum of 10 pg of Ni on a Si-wafer. Trace elemental analysis for low-Z elements is also very important in Si wafer surfaces and synchrotron radiation has been used for total reflection x-ray fluorescence analysis (TXRF) to measure concentrations of Na, Mg and Al impurities. These atoms are very difficult to detect with conventional x-ray sources as the x-ray emission energies are far higher than the element's absorption edges, and also due to interference from heavier element's overlapping secondary absorption edges. It was reported that using synchrotron radiation, the detection limits for Mg are 0.07 pg / 1.7 x 109 atoms/cm-2 compared to 90 pg / 2.3 x 109 atoms/cm-2 for conventional Cr-tube xray sources and 7 pg / 1.8 x 1011 atoms/cm-2 for Si-tube x-rays. Trace elemental concentrations of Na, Mg and Al in Si wafer surfaces were thereby measured using TXRF and lower detection levels of 200, 125 and 500 fg were obtained respectively. Angle-dependant TXRF was also used to measure depth profiles of Al impurities in Si wafers in the range 5 – 20 nm. (C. Streli et al, Spectrochimica Acta Part B 54 (1999) 1433 - 1441) XRF has also been used to perform trace elemental analysis on large synthetic diamonds grown in Japan from metallic solvents. Commonly incorporated metallic impurities are Cr, Mn, Fe, Co and Ni and these have received great attention for obtaining impurity controlled crystals. In one article, measurements are described in which the concentrations of Co in synthetic diamonds were compared for diamonds grown with different starting conditions, and XANES measurements were also performed to identify the lattice positions which the Co impurities occupy within the diamond matrix. (S. Hayakawa et al, Journal of Crystal Growth 210 (2000) 388 – 394) XRF measurements were made on aerosol particles found in air samples obtained from an urban site in Switzerland. For the evaluation of the influence of atmospheric aerosols on human health (eg the toxicity of a material when inhaled) and the dispersion via atmospheric transport, knowledge about the composition and size distribution is required. Such particles (around 0.1 – 1.0 μm in diameter) deposit slowly within a few days of precipitation and can therefore be transported over long distances, having effects in regions remote from the source. Furthermore, particle size and shape are key factors that control the extent of the penetration into the human respiratory system. Samples of air from central Zürich were analysed to measure via XRF the concentrations of 23 elements within aerosol particles of various sizes in the range 0.1 – 1.0 μm. Elements with Z = 13 – 24 were measured at the Swiss Light Source whereas elements with Z = 25 – 82 were measured at beamline L at Hasylab. Eight different sources were identified for the particles – secondary sulfate, wood combustion, fireworks, road traffic, mineral dust, de-icing salt, industrial and anthropogenic activities and many conclusions could be made about the distances travelled and daily variation of their concentration levels. (A. Richard et al. Atmos. Chem. Phys., 11 (2011) 8945 - 8963) XRF has been used for biological studies, for example in the investigation of the causes of Alzheimer's Disease (AD) which is characterised by the misfolding and plaque-like accumulation of a naturally occurring peptide in the brain called amyloid beta (Aβ). The Aβ peptide ranges in length from 39 - 43 animo acids, and in AD plaques it is the major constituent. These plaques are formed due to the transformation of Aβ from a soluable form to an aggregated, fibrillary structure that is neurotoxic and there is increasing evidence that interactions between Aβ and metals such as Cu and Fe play a role in this transformation. It is thought that the strongly redox-active Aβ reduces Fe3+ and Cu2+ which produces H2O2 in the presence of oxygen. H2O2 is a source of toxicity in neuronal damage, however it has also been found that the presence of the redox-inert Zn binds the Aβ, preventing the reduction of Cu2+ and reducing the formation of H2O2. Therefore a direct anaylsis of Aβ and metal ions in AD patients is essential to fully understand this process of AD development and for potential future preventions and treatments. XRF was used to image trace-elements of Ca, Fe, Cu and Zn in areas of brain tissue from patients with confirmed AD, which had been previously imaged with synchrotron Fourier transform infrared microspectroscopy (FTIRM – which is used for imaging fibrillary Aβ deposits in brain tissue). A homogeneous distribution of Zn, Cu, Fe and Ca were found in the AD brain tissue, exhibiting “hot spots” of high metal content. A strong correlation was found between Cu, Zn and Aβ location which are consistant for a protective role for Zn2+ in AD, where plaques form as the result of an antioxidant response for the underlying oxidative attack. These results and further studies involving analysis of earlier stages of the disease will be useful in accessing the sequence of events that leads to co-localisation of Cu, Zn in Aβ plaques in AD. (L. Miller et al., Journal of Structural Biology 155 (2006) 30 - 37) A second group has performed trace element distribution analysis in the brain sections of rats using XRF with the aim of measuring the effects of iodine deficiency on concentrations of trace elements of P, S, Cl, K, Ca, Fe, Cu, Zn, Br, Rb in the thalamus, cerebral cortex and hippocampus. The study found several interesting effects, for example that Zn is concentrated along the hippocampal fissure in rat brain and that iodine deficiency leads to marked alterations in the spatial distribution of Zn and Ca during brain development stages, but more experiments were planned to confirm and explain such observations. (Liu et al., Spectrochimica Acta Part B 59 (2004) 255 - 260) Trace element analysis using synchrotron XRF also finds application in forensic science, for example the analysis of minute glass fragments found at crime scenes which can serve as one of the most important evidentiary materials in identification of the culprit in criminal cases such as hitand-run, theft and murder. Measurements were made at the Japanese synchrotron and the lower limits of detection for the elements of Ca, Fe, Sr, Zr, Sn, Ba, Ce, Sm, Hf were measured and range from 0.5 – 50 ng in a spot-size smaller than the beam diameter of 500 μm x 500 μm. Small fragments of six different types of glass were successfully discriminated using this method demonstrating the power of XRF in forensic science. (T. Nakanishi et al., Forensic Science International 175 (2008) 227 - 234) XRF is also used in ecological studies, for example in the study of the life histories of yellow and silver eels collected in marine and fresh water in Asia which can provide a key to understanding the evolutionary process of fish migration as well as providing a record of changes in the elements present in the environment during a fish's lifespan. This is performed by analysis of the inner ear of a fish which contains two tiny particles of CaCO3 called otoliths which function as the organ of equilibrium. The otoliths increase in size as the fish age, and the water environment is the source of Ca and CO3 that constitute their makeup. It is thought that CaCO3 precipitates together with traceelements from the environment during daily growth increments. It is known that the Sr content of seawater is more than 100 times higher than that in freshwater, therefore Sr/Ca ratios were measured for common eels found at different locations. It was observed that the otoliths of a typical Japanese eel found in the Uono river have a Sr-rich core and a Sr-deficient region in the outer layers which indicates the juvenile fish migrated from the sea to fresh water for the remainder of its life. Conversely, all specimens collected in the East China Sea and the North Sea show a secondary peripheral region of elevated Sr which was not observed in the river specimens, indicating that the fish did not spend their juvenile and adult lives in freshwater. It is thought that sea-residency of freshwater eels would occur more frequently at higher latitudes where the food availability in freshwater habitats is lower than in adjacent coastal regions. (I. Nakia et al, Spectrochimica Acta Part B, 54 (1999) 167 - 170) Figure 4: 2D XRF imaging of trace elements in otolith. The XRF intensity of each element was normalised to that of Ca with black being highest and white the lowest intensities. (Left) Sr/Ca and (centre) P/Ca ratio of an otolith of an eel collected at Uono river and (right) Sr/Ca ratio of otolith of an eel caught in the East China sea. A popular use of XRF analysis is for the study of ancient and historical items. XRF is used for elemental analysis of specimens while X-ray absorption spectroscopy (XAS) gives information on local structure around selected elements. These methods have been combined to perform investigations on items of cultural heritage (paintings, statues, ceramics), for example: ◦ ◦ ◦ ◦ ◦ ◦ identification of materials used, how to preserve them to detect fraud or alterations to a specimen analysis of inks used in a particular area and time period origin of stones used in statues techniques used in early steel making restoration of faded/erased ink in old manuscripts Industrial uses of synchrotron XRF in the rapidly growing field of nanotechnology include determination of the distribution of elements in electronics, products from energy company, building materials, plastics, cosmetics, wood and pulp, foods, pharmeceuticals and biological structures. X-ray Absorption Spectroscopy X-ray Absorption Spectroscopy (XAS) and the related X-ray Absorption Near Edge Structure (XANES) and Extended X-ray Absorption Fine Structure (EXAFS) are useful tools for characterising the local environment (coordination number and interatomic distance) and the electronic state of an element within a sample of interest. These techniques find application in the development of catalysts for a very broad range of chemical reactions. The ultimate goal is to obtain significant structural and electronic characteristics which can be correlated to the catalytic activity in a given reaction. One example has been the analysis of Co based catalysts for Fischer-Tropsch synthesis which is a series of chemical reactions that convert a mixture of CO and H into liquid hydrocarbons and produces a synthetic lubrication oil and synthetic fuel, typically from coal, natural gas or biomass. Several studies have been focussed on the catalytic nature of Co species using many experimental techniques but little has been concluded about the electronic state of the Co ions, even though this is a key issue in understanding the mechanism of the CO hydrogenation. In one study, Co containing catalysts were analysed using L-edge XANES and the absorption spectra were compared to those obtained for several Co containing reference samples. It was found that one catalyst sample was associated with the presence of large clusters of Co3O4 whereas another had an amorphous structure similar to Co2SiO4. Heating of the Co3O4 containing sample in a reducing atmosphere up to 500 °C resulted in a reduction of the Co3O4 clusters as well as a disappearance of the fine structure of the Co absorption spectrum which could be caused by disorder in the first coordination sphere of Co atoms. The significant information gained from this particular result can be utilised to link structural characteristics to the catalytic activity as well as being a challenge for theoretical physics. A further example was the investigation of the environment of sulfur in organic substances in experiments designed to emulate the maturation of organic matter in petroleum reservoirs at moderate temperatures over geological time scales. These emulations are performed at high temperatures for a faster maturation time, and in the case of iron containing kerogenes (organic chemical compounds that make up a portion of the organic matter in sedimentary rocks and are a source of fossil fuels) the quantity of H2S may be increased or decreased due to a number of factors and so sulfur K-edge XANES was used to fingerprint the forms of sulfur in these organic compounds. Elemental analysis and percentage of sulfur in different forms were thereby measured and some insight was given into the processes governing hydrocarbon formation in petroleum reserves. (D. Bazin et al, Applied Catalysis A: General, 213 (2001) 147 - 162) XAS was also used to investigate the environments of Cr, Cu and Zn in corroded and uncorroded aluminium alloys used in aerospace frames, skins and structural members. It is of interest to extend the life of aerospace components as much as possible, so samples of an aluminium alloy (7175 T736 aluminium) were analysed with XAS in an uncorroded form as well as a sample which had been stored for 9.8 years in an uncontrolled environment. It was found that only the Cu site significantly changed during corrosion, however no chemical shift was observed and nor was the presence of Cu compounds found. Using XANES and EXAFS it was concluded that about 20% of the Cu atoms sampled was present as disordered, metallic Cu after corrosion, and furthermore in the surfaces of corroded areas, high concentrations of Al2O3/Al(OH)3 are present. These findings are consistent with galvanic corrosion due to the presence of water. (R. Greegor et al. Corrosion Science, 39, 12 (1997) 2095 - 2116) XANES spectroscopy was also used in the analysis of rubber tyres in which the state of sulfurcrosslink distribution and crosslink density can be characterised. These arise during the vulcanisation process and oxidative-aging processes take place at the cross-links. (Hormes et al Nucl. Inst. Meth. A 467-468 (2001) 1179 – 1191) XAS was used in conjunction with XRD and XRF to investigate the chemistry of yellow ancient tile glazes (17th – 19th century) which have deteriorated due to atmospheric conditions and should be restored, therefore a concise knowledge about the basic materials and colourants is used so that only conformable new products are used. The measurements were able to show the presence of Pb and Sb in fragments of tile glazes and the general conclusion about the constitution of the yellow glazes of the ancient tiles is that Pb is hosted in the glassy matrix while the actual colouring agent being a dispersed Sb-pyrochlore phase. (M. Figueiredo et al, Nucl. Inst. Meth. B, 238 (2005) 134 –137) The famous Maya blue pigmentation developed by the Mayan people has been studied in depth due to its brilliant Caribbean sea blue colour which has exhibited remarkable durability for over 1000 years despite exposure to the harsh environmental conditions of the Yucatan peninsula and is resistant to acids, alkalis, organic solvents and biological degradation. This pigmentation has been emulated in modern times by heating a mixture of palygotskite clay with indigo at 150 °C for two days which produces a Maya blue color with identical physical and chemical properties to field samples. It is of interest to study these systems not just for the ability to produce blue pigmentation from environmentally safer starting materials, but also to gain insight to the technology transfer within the Mayan civilisation. Samples of ancient and synthetic samples were analysed with XANES and EXAFS to study the chemical nature of iron and iron nanoparticles detected using Transmission Electron Microscopy. It was found in that in authentic Maya blue samples Fe3+ is the dominant oxidation state and one sample contained 25% Fe2O3 + 75% FeO(OH) and approximately 100% FeO(OH) in a second. Simulation analysis of Fe EXAFS from the latter sample found a disordered structure in which coordination spheres with noticeable differences from those observed in authentic FeO(OH). It was concluded that the Fe oxide phase present in this sample is probably incompletely hydrated and is transitional from Fe2O3 to FeO(OH). The original Maya blue may have contained only Fe2O3 that was converted to FeO(OH) due to heat and ambient moisture in the Yucatan region. (L. Polette et al, Microchemical Journal, 71 (2002) 167 - 174) Industrial uses of synchrotron absorption spectroscopy in the rapidly growing field of nanotechnology include analysis of surfaces and local chemical environments in electronics, products of the metallurgy industry, functional glasses and glass-ceramics and plastics; aging processes in products from energy companys and building materials. Characterisation of catalysis processes in petrochemistry is also of interest. Synchrotron X-ray Diffraction (XRD) X-ray diffraction (XRD) using synchrotron radiation is advantageous over conventional x-ray sources as higher resolution patterns can be obtained which can be used to resolve more difficult space group or symmetry problems, or can more easily identify minority phases present in samples. Structural properties of Mg and Mg alloys are of interest due to their potential application as lightweight materials for air-space, aviation and automotive-industry applications, for example Volkswagen proposed a prototype of a weight reduced car (about 290 kg) with much lower gasoline consumption. To begin such a project, several different types of Mg alloy and Mg reinforced composites were prepared from Mg grains of size 30 μm compacted into cylindrical rods and global and local texture measurements were made using synchrotron XRD at DESY. (H.-G. Brokmeier et al, ICDD 2003 – Advances in X-ray Analysis 46) Further examples of studied materials and applications of XRD: The Boeing Corporation used synchrotron XRD to develop lightweight components in the form of polyethyl ether Ketone resins substituted with Al with no strength loss, resulting in significant weight reductions in its 757 aircraft. Chemical companies use synchrotron radiation to investigate the synthesis and properties of new catalysts which are used in almost all chemical engineering processes. Fine particles can be observed down to the nanoscale which is useful in the study of chemical processes such as combustion in real time. Two examples of this are: Analysis of Ni exchanged zeolite Y (a catalyst for production of benzene from acetylene) which needs to be heated to 200 °C for approx. 2 hours to begin to catalyse. High resolution XRD was performed on the sample during the heating process and the migration of Ni 2+-ions from a catalytically inactive lattice site to a catalytically active “supercage” site was observable (using Rietveld refinement) which demonstrates not only the power of the powder diffraction technique but also how physical chemists are approaching a better understanding of the catalytic process. Analysis of rare-earth doped oyrochore catalysts used in converting CO2 and CH4 greenhouse gases into useful chemical feedstock. Synchrotron powder diffraction is very useful for rapid diffraction data capture on time dependant systems, such as hydration of various systems. For example, a study on hydration properties in cement mixes was performed in which diffraction date was obtained every 10 s on a “mini cement mixer” setup in the experimental station and conclusions were able to be made about the rate at which the main hydration product in cement (a calcium sulfo-aluminate hydrate or “ettringite”) starts to form (ie within the first 10 s of mixing). The unit cell a-parameter of this crystal phase observed to increase by a factor of 0.7% over a longer time period which is consistent with a substitution of 50 - 70% of the sulfate groups by hydroxy/carboxy groups, demonstrating how such measurements can be used to clarify the nature of complex hydration processes. (R. Cernik et al, Rad. Phys. Chem 45 (3) (1995) 445 - 457) More recently, hydration processes of ordinary portland cement (OPC) have been investigated using high-resolution synchrotron XRD to analyse the early formation of ettringite and the effects of additives such as retarding admixtures and superplasticisers (which optimise the flow properties of cement and reduce the water/cement ratio providing higher compressive strength) on the millisecond timescale. These additives have been found to be most influential during the first minutes of hydration. Measurements were made on pure OPC as well as OPC containing polycarboxylate ethers (PCEs) with differing charge densities. PCEs are commonly used superplasticisers with strong dispersion effects which arise from charge and adsorption effects during the cement hydration. During the measurements, XRD measurements were performed every few milliseconds and the (100) diffraction peak of ettringite was observed for each sample. It was found that in pure OPC, the concentration of ettringite increases exponentially over the whole time of hydration whereas for those containing PCEs, the rate of increase is significantly decreases while liquid water remained in the mixture and was not absorbed by the cement. Using this method, a model of the inhibition of the ettringite formation process by PCEs was able to be formulated. (M.-C. Schlegel et al, Angew. Chem. Int. Ed. 51 (2012) 1 - 5) Industrial uses of synchrotron XRD in the rapidly growing field of nanotechnology include analysis of stress and detection of point defects in electronics, detection of stress gradients and nanoclusters in alloys, local crystalline ordering in advanced glasses and glass-ceramics, phase identification and corrosion processes in new products from energy companies, identification of deposits and colloidal particles in oils and observations of chemical transformation and crystallisation in petrochemistry. Nanomorphology of gels and emulsions for cosmetics and their effects in tissues on the molecular level are also of interest, as well as stability and aging of foods and protein-drug interactions. Studies of crystals with large unit cell dimensions (biological molecules) Pharmaceutical companies use synchrotron radiation for the determination of biological structures ranging from small peptides to the ribosome to entire viruses which is essential for the understanding of biological processes and for developing treatment of diseases and the design of many drugs. Brilliant synchrotron sources offer major advantages over laboratory sources for the study of crystals with large cell dimension (eg exceeding 1000 Å). Using protein crystallography techniques,the crystal structures of more than 61840 proteins, nucleic acids and other biological molecules have been determined, compared to the next most widely used technique, nuclear magnetic resonance, which has resolved 8759 chemical structures. For example, the structure of many viruses have been solved at high resolution, eg the human rhino virus 3 at 3.0 Å resolution, the human rhino virus 16 at 2.15 Å resolution, the bacteriophage Qβ at 3.5 Å resolution, the simian virus 40 at 3.1 Å resolution among many others. (K. Moffat et al, Current Opinion in Structural Biology 7 (1997) 689-696) UV-VIS Spectroscopy Dedicated synchrotron generated UV beamlines like SUPERLUMI at DESY is heavily used in the development of luminescent materials used for radiation detectors, new light sources, lamp phosphors and persistant phosphors. Such measurements are required for the understanding of intrinsic and extrinsic luminescent mechanisms and optimisation of luminescence characteristics such as luminescence intensity, scintillation lifetime and radiation stability. New scintillator materials for x-ray and gamma detection are constantly being tested and many scintillator crystal companies have a need for optimisation of their materials which can be performed at stations like SUPERLUMI. Summary Synchrotron radiation has a very wide range of industrial application, ranging from trace-elemental analysis in Si-wafers used in the semiconductor industry using XRF, to quality control in aircraft components using x-ray microtomography, XRD and XAS to real time analysis of structural changes in materials using XRD, the use of protein crystallography for structural analysis of a host of biological molecules and many examples of the analysis of ancient and historical materials. The first industrial partners that should be contacted are therefore members of the aerospace, aviation and automotive industries, the oil industry, manufacturers of electronics, pharmaceutical companies and perhaps to a lesser extent cement manufacturers, forensics labs, medical research companies, manufacturers of luminescent materials. Graham Appleby 19.04.2012