Dr Keshaw Prasad Shrivastaw
advertisement
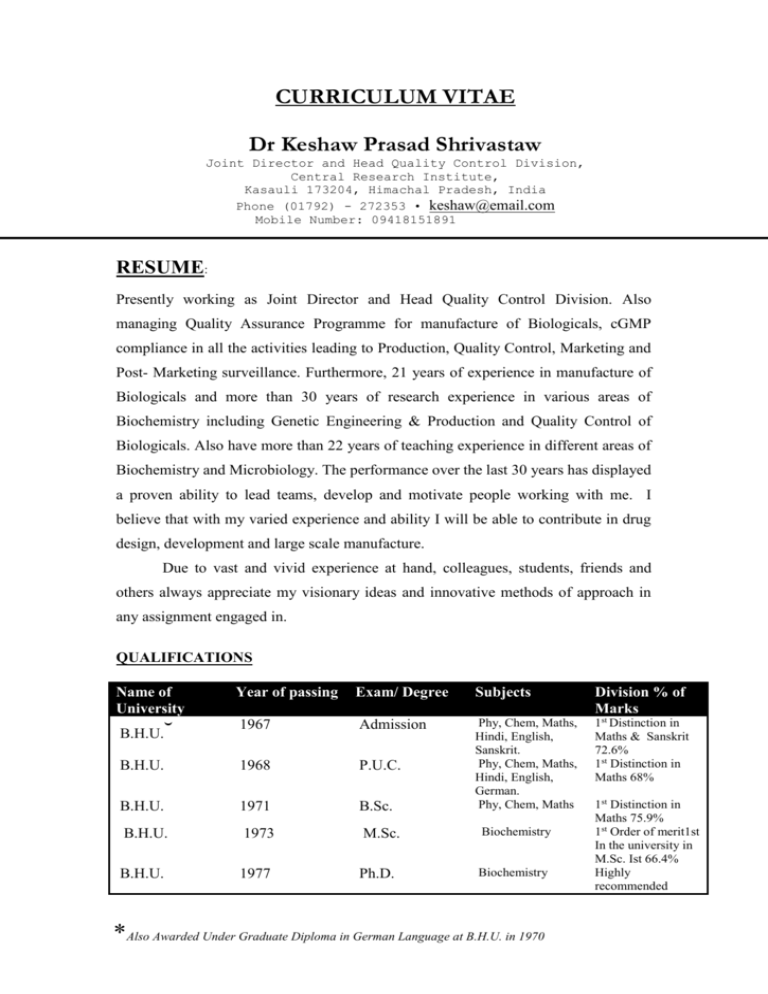
CURRICULUM VITAE Dr Keshaw Prasad Shrivastaw Joint Director and Head Quality Control Division, Central Research Institute, Kasauli 173204, Himachal Pradesh, India Phone (01792) - 272353 • keshaw@email.com Mobile Number: 09418151891 High Tech Sales Manager (international background 2 pages) Igor Slovak RESUME: Presently working as Joint Director and Head Quality Control Division. Also managing Quality Assurance Programme for manufacture of Biologicals, cGMP compliance in all the activities leading to Production, Quality Control, Marketing and Post- Marketing surveillance. Furthermore, 21 years of experience in manufacture of Biologicals and more than 30 years of research experience in various areas of Biochemistry including Genetic Engineering & Production and Quality Control of Biologicals. Also have more than 22 years of teaching experience in different areas of Biochemistry and Microbiology. The performance over the last 30 years has displayed a proven ability to lead teams, develop and motivate people working with me. I believe that with my varied experience and ability I will be able to contribute in drug design, development and large scale manufacture. Due to vast and vivid experience at hand, colleagues, students, friends and others always appreciate my visionary ideas and innovative methods of approach in any assignment engaged in. QUALIFICATIONS Name of University Year of passing Exam/ Degree 1967 Admission B.H.U. 1968 P.U.C. B.H.U. 1971 B.Sc. B.H.U. 1973 M.Sc. B.H.U. 1977 Ph.D. ˘ B.H.U. Subjects Division % of Marks Phy, Chem, Maths, Hindi, English, Sanskrit. Phy, Chem, Maths, Hindi, English, German. Phy, Chem, Maths 1st Distinction in Maths & Sanskrit 72.6% 1st Distinction in Maths 68% Biochemistry Biochemistry *Also Awarded Under Graduate Diploma in German Language at B.H.U. in 1970 1st Distinction in Maths 75.9% 1st Order of merit1st In the university in M.Sc. Ist 66.4% Highly recommended Dr K.P.Shrivastaw EXPERIENCE Engaged in Production and Quality Control of Biologicals produced at Central Research Institute since 1983. The production targets of several million doses of these biologicals are released for various immunization programs in the country. 1. Quality Control & Quality Assurance Functions Carrying out the Quality Control and Quality Assurance functions at Central Research Institute. These functions are very important and form absolute prerequisites for TQM in any pharmaceutical industry. It is generally agreed upon that TQM in any organization is a long term continuous evolving process Various productions units are inspected and suitably advised to improve upon and maintain cGMP in the institute’s different functions. Conversant with the latest VALIDATION procedures viz. DQ, IQ,OQ ,CQ, PQ and also Process Validation. Approved and authorized more than 300 SOP's. Licensing Procedures for the production of Immunobiologicals handled for the last 09 years and also coordinated the inspections by outside agencies Drugs Controller/ WHO. Quality Control testing methods requiring animals have been reviewed in order to reduce the usage of animals. Training programs were arranged for staff working in this institute as well as those coming from other institutes of the country and abroad (WHO fellow) from time to time. 2. Six Sigma Six Sigma at many organizations simply means a measure of quality that strives for near perfection. In fact, Six Sigma is a disciplined, data-driven approach and methodology for eliminating defects (driving towards six standard deviations between the mean and the nearest specification limit) in any process -- from manufacturing to transactional and from product to service. In short, it is a quality methodology that can produce significant benefit to businesses and organizations. I am well aware of the six sigma concept in the industry. 2 Dr K.P.Shrivastaw 3. Other Activities 4. i) NEW PROJECTS- Vision ii) Pollution control activities iii) Administrative Control, Accounts and Stores Management R & D Activity Have 30 years of research experience in different areas of biochemistry viz. Enzymology, molecular biology, nutritional biochemistry, neurochemistry, Genetic Engineering and Production & QC of Biological at different institutes of the country and abroad e.g. B.H.U., Central University of Hyderabad, University of Paris & University of Rennes , France, HP University Shimla at Central Research Institute, Kasauli. Published 22 outstanding research papers in national and international journals of repute and 9 are ready for publication. Under R & D effort, designed and developed manufacturing techniques for the manufacture of new vaccines e.g. Meningococcal vaccine and Acellular pertussis vaccine. Introduced new and innovative coordinated management methods which have resulted in increased production in a time bound manner. Developed new QC Testing methods. These New Methods developed for quantitation of thiomersal and formaldehyde in Biologicals (Published in Biologicals, U.K., 1995) is used routinely in the institute and has now been recommended and included in Indian Pharmacopoeia recently in 2003. 5. Awards Received Awarded JRF, SRF and Post Doctoral Research fellowship of C.S.I.R., New Delhi India, during my Ph.D programme. Awarded Merit certificate by B.H.U for standing first in the university M.Sc. Ist (Biochemistry). Awarded INSA fellowship to visit West Germany in the area of Genetic Engineering in 1989-90. Awarded Post Doctoral fellowship by INSEREM and CNRS, France in the area of Genetic Engineering and Molecular Brain Biochemistry in 1979-1980 and 1985 to work with Prof. Ph. Chevaillier & Prof. M. Philippe at Univ of 3 Dr K.P.Shrivastaw Paris and Univ of Rennes, France on different projects using various advanced techniques in Genetic Engineering. Awarded Post Doctoral fellowship to work with Prof. M.V. Viola at the University of Connecticut, Farmington, Connecticut, U.S.A. in 1980. 6. Memberships Of Distinguished Academic Bodies Enrolled as Life Member/Full Member/Founder Member of various National and International Scientific Bodies. 7. National and International Conferences and Workshops Attended several national and international conferences in various areas of Biochemistry , Microbiology and manufacture of biologicals. 8. Teaching Experience Have 22 years of teaching experience in teaching programmes of Graduate and Post Graduate Students of Bio-chemistry & Microbiology at different Institutes of the Country, since July, 1978 till date. 9. Other Information Well conversant with USP, EP, BP & IP and latest development in Quality Assurance functions in pharma sector. FDA APPROVED " MANUFACTURING CHEMIST / ANALYST" Date of Birth- 7th January, 1952 Languages: English ( Fluent), German, French, Sanskrit and Hindi In possession of a valid passport. Ready to take up assignments in different countries and can handle different projects simultaneously. Health: Gained Experience in various Yoga Techniques to solve health and other life related problems. Possess Himachali Domicile. (Dr.K.P.Shrivastaw) 4







