Guided Inquiry Lab #3: Identification of an Unknown Cation and
advertisement

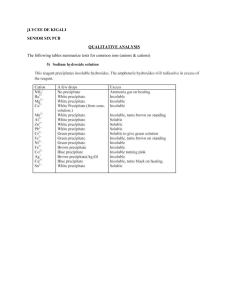
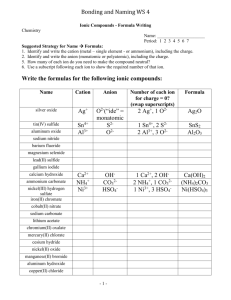
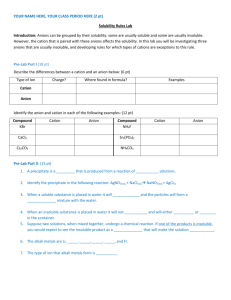
Guided Inquiry Lab #3: Identification of an Unknown Cation and Anion by Qualitative Analysis Purpose: To identify an unknown compound found in an unlabeled chemical container this summer in the back of Mr. Thomas’ chemical cabinet using a series of chemical tests and qualitative observations. Introduction: Qualitative analysis has long been a fundamental practice in research chemistry. Entire books have been written to detail the various experiments and tests that can be used to identify the presence of certain cations, anions, and even types of organic molecules. In this experiment, you will be given the opportunity to develop your own procedures to identify an unknown cation and anion in a series of aqueous mixtures. You will have access to pH paper, concentrated sulfuric acid, conc. FeSO4(aq) and 1% hydrogen peroxide (H2O2) in water, along with any other materials requested from the laboratory teacher. The following is a suggested list of tests, but is not all inclusive. If identification cannot be made after performing these tests, further identification procedures will need to be researched and performed. Brown-ring test: This test is used to identify the presence of nitrate, NO3–. Add a small amount of the test solution to ~1 mL of conc. iron(II) sulfate in a small test tube. Then, using a transfer pipet, slowly add concentrated sulfuric acid to the test tube. The sulfuric acid will form a second layer (it is far more dense than the FeSO4 solution), and at the interface of the two layers, the appearance of a brown ring signifies the presence of NO3–. The brown ring is actually trapped NO(g), which is produced through an oxidationreduction reaction with the Fe2+: NO3–(aq) + 4 H+(aq) + 3 Fe2+(aq) NO(g) + 3 Fe3+(aq) + 2 H2O(l) The solution should also turn slightly yellow, as Fe3+ complexes with water to form yellow Fe(OH2)63+. pH Test: You will have access to pH paper to test the pH of the various solutions. Flame Test: When metal ions are heated in a flame, they give off a characteristic color due to the excitement of certain electron transitions. By dipping a nichrome wire into a solution and placing it in a Bunsen burner flame, you can observe the colors. Ammonium Test: One can test for the presence of ammonia by adding sodium hydroxide to the solution in question. The hydroxide will pull a hydrogen ion off of the ammonium to form ammonia: OH–(aq) + NH4+(aq) NH3(g) + H2O(l). The formation of ammonia can either be detected by its pungent smell, or by holding a piece of damp acidic (red) pH paper above the solution. Solubility Tests: One of the best ways to identify the presence of certain cations and anions is to look for the formation of precipitates as certain combinations of cation and anion are mixed. There are two ways for you to determine which tests to perform. One is to randomly test all combinations of cations and anions, the other is to use a solubility chart from either your book or the web to narrow down the tests to specific combinations of ions that might assist in their identification. Iodine Test: In acidic solution, hydrogen peroxide can oxidize I– to form I2, which then reacts with another I– to form I3–, which has a yellow-brown color: 2 I–(aq) + H2O2(aq) + 2 H+(aq) I2(aq) + 2 H2O(l) I–(aq) + I2(aq) I3–(aq) This test can be performed by adding a some HCl to the solution in question and then adding some H2O2 dropwise with a transfer pipet. Prelab: 1. Before you come to the lab on the first day, you should get together with your labmates and develop a strategy for the tests you will do on the first day. Also, you will need to look up the solubilities of various combinations of the cations and anions to determine which combinations might be useful in identification. Remember, nitrate, ammonium, and hydrogen ions will not form precipitates with anything. 2. Before coming to lab on the second day, you should prepare a procedure for identifying the cation and anion based on the first day’s tests. Materials: well plate dilute nitric acid solution test tubes sodium carbonate solution transfer pipets potassium hydroxide solution pH paper ammonium chloride solution niochrome wire 1% hydrogen peroxide solution Bunsen burner concentrated sulfuric acid solution iron(II) sulfate solution concentrated iron(II) sulfate solution Procedure: In this experiment, you will be performing and recording your own procedures. Be sure that whoever is reading your report can actually perform each step recorded as you have it written. Be brief, but thorough…short, sweet and potent! Pre-lab Questions: 1. Hydrogen ions will never register any color in a flame test. Why is this? 2. If you look at the list of cations and anions that form precipitates, two striking features are clear. First, it is very rare to find a precipitate formed by a +1 cation and a –1 anion (Ag+ and Hg22+ being the exceptions). Second, as you move up the periodic table in a group (say the alkaline earth metals, Ba through Mg) the precipitates they form become less and less soluble. What is the explanation for this? 3. Even though sulfate is the conjugate base of a weak acid (HSO4–), it does not register as basic in a pH test. There are two very good explanations for this (one mathematical, the other practical). What are both explanations?