Lactase Enzyme Lab
advertisement

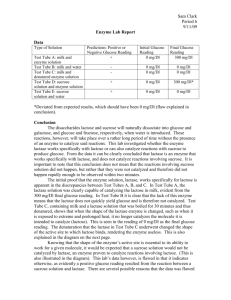
Lactase Enzyme Lab Exp. #1 Kathy Bui Per. 4 DUE: 9/3/09 1 Background Info: Lactose, the sugar found in milk, is a disaccharide composed of glucose and galactose (both six-sided sugars). Sucrose, ordinary table sugar, is also a disaccharide composed of fructose and glucose. Glucose is a six-sided sugar and fructose is a five-sided sugar. Lactase is an enzyme that breaks lactose down into galactose and glucose. Sucrose is similar to lactose. Skimmed milk is not going to remove the lactose, as the lactose is not contained in the fat of the milk, but in the whey. Lactose intolerance is caused by some people's inability to produce the enzyme lactase. Lactase is naturally occurring in unpasteurized milk. Pasteurization removes the natural lactase and can make it difficult to digest any pasteurized cheese products. Hypothesis: Type A. Test tube with skim milk and enzyme solution. B. Test tube with skim milk and water. Initial Final (300) (300) 0 (mg/dl) 3000 (mg/dl) Skim milk being a dairy product contains a lot of lactose, but no glucose because it is all in a disaccharide form. When adding an enzyme solution, lactose should break down into glucose and galactose. 0 (mg/dl) Skim milk being a dairy product contains a lot of lactose, but no glucose because it is all in a disaccharide form. When adding water, the lactose substance should not break down because water does not contain and enzymes (lactose). 0 (mg/dl) Reasoning C. Test tube with skim milk and denatured enzyme solution. 0 (mg/dl) 0 (mg/dl) Skim milk being a dairy product contains a lot of lactose, but no glucose because it is all in a disaccharide form. An enzyme solution is denatured when the protein molecule loses its proper shape and cannot function properly. In saying so, when adding a denatured enzyme solution, the lactose within the skim milk should not break down. D. Test tube with sucrose solution and enzyme solution. 0 (mg/dl) 3000 (mg/dl) Because sucrose is also a disaccharide like lactose, when an enzyme solution was added, the disaccharides should break into glucose and fructose. E. Test tube with sucrose solution and water. 0 (mg/dl) 0 (mg/dl) Pure water contains no enzymes, in saying so, if water is added to a sucrose solution there should still be no presence of glucose. 2 RAW DATA: 3 DATA: Type Result Initial (300) Final (300) A. Test tube with skim milk and enzyme solution. Glucose was present after enzyme solution was added. 0 (mg/dl) 1000 (mg/dl) B. Test tube with skim milk and water. No glucose was present after water was added. 0 (mg/dl) 0 (mg/dl) C. Test tube with skim milk and denatured enzyme solution. No glucose was present after denatured enzyme solution was added. 0 (mg/dl) 0 (mg/dl) D. Test tube with sucrose solution and enzyme solution. No glucose was present after enzyme solution was added. 0 (mg/dl) 0 (mg/dl) E. Test tube with sucrose solution and water. No glucose was present after water was added. 0 (mg/dl) 0 (mg/dl) *Note: Trials A, B and C eventually changed into a greenish color most likely because when milk is left out at room temperature eventually denatured itself. Data Analysis: Our results led us to believe that in only one of the five present trials was there any reaction relating to the breaking of disaccharides. When an enzyme solution was added to the skim milk, the presence of glucose changed from 0 to 1000, suggesting that their must have been some kind of reaction even though it is not viewable by the naked eye. When water was added to the skim milk, glucose was not recorded to be present, suggesting that the lactose within the milk was not broken down. Also, the addition of trial C suggests that unlike the enzyme solution, the denatured enzyme solution did not break down the lactose in skim milk. When the enzyme solution was added to sucrose, we also recorded no change in the number of glucose. It is expected that the last trial, trial E, had no resulting change in the number of glucose because pure water, containing only H20 attains no enzymes to break down any disaccharides. 4 Conclusion: In our hypothesis we stated that there is no presence of glucose because in both cases of skim milk and sucrose, the glucose molecules are bonded with another molecule forming a disaccharide. When adding water to these substances, there should be no change in the molecular structure because both sucrose and skim milk are non-polar. Also, water contains only H20 molecules, there are no enzyme substances present in pure water hence trials B and E attains no change in the number of glucose. Denatured enzyme solutions are enzyme solutions that have been under the influences of high temperatures, extremes of pH, heavy metals, and alcohol. As we have learned in class, enzymes function better at specific temperatures of pH’s. So denatured enzyme solutions will not function properly at higher temperatures let alone at all because the molecule loses its shape. Hence, trial C had no change in its number of glucose. Trials A and D had enzyme solutions added to them which led us to believe that the number of glucose should grow drastically when lactase is added to the skim milk and sucrose. Although we were correct in saying that it would grow due to our prior knowledge of lactase’s function, the number of glucose in skim milk did not increase drastically and the number of glucose in sucrose did not change at all. In trial A, we forgot to take in account that skim milk is not made of 100% lactose but many other substances so the general ratio of glucose may not be that great in the solution when the disaccharides are broken. In trial D, we did not see any resulting increases in glucose because lactose is similar to sucrose but, lactase will break down only lactose because of the shape of the sugar. 5 Limitations Improvements 1. Contamination: many students were using 1. Because the procedures did not clearly the same sources so contamination may state that contamination within this have occurred by accidentally using the experiment was clearly a factor, the act of wrong droppers, etc. 2. The milk we used was left out of the refrigerator for a period of time which may have caused the milk to become denatured in the process and may have affected the experiment. 3. contamination may have occurred and should be added to the procedure. 2. In the procedure, a step or a note should be added reminding future students to place the milk back in its proper place. In my group personally, we did not fully 3. The instructions would be helpful if understand how the glucose test strips changed into saying that the strips should worked. So we had ended up making a be dipped into the solution, taken out and mistake when testing for glucose. left there for 2 minutes before recording Although the procedure instructs us to the results of the test. “time for two minutes and test for 1. More efficient strips or the basic strips glucose.” we were not sure if that meant leave the strips in the solution for two minutes or dip the strip in and wait for two minutes. We ended up leaving the strip in for a long period of time. 4. The labeling system on the glucose testing that tell you whether it is a positive (yes) or negative (no) in regards to whether there is or is not any glucose would have been a much easier and more effective process because in the group I worked strips was also very hard to decipher. At with, the focus was mainly directed at one point, we all got very caught up in what color is the strip rather than why is fighting over what color the strip was that strip the color that it is. actually portraying. 6