Port Said International Schools Worksheet
advertisement

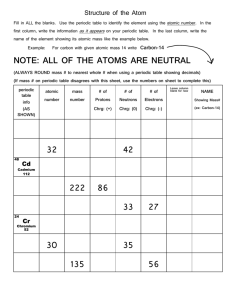
Port Said International Schools Better education for future generations Science Department Worksheet: 2 Grade: 8 Name:……………………………. A) Choose the correct answer : 1-The atomic number of an element which lies in group ( 4A) and period(2) is …….. ( 4 - 5 - 6 - 14 ) 2-……………. discovered the energy levels. (Mosley - Mendeleev - Bohr - Rutherford) 3-The atomic size of the elements in the same period ……….by increase of their atomic number. (increases - decreases does not change - no correct answer ) 4-The electronegativity of fluorine element is equals………. ( 2 - 4 - 1.5 - 9) 5-By increasing the atomic number of elements in the same group, the electronegativity……… ) increases decreases does not change - no correct answer( B) What is meant by : Electronegativity? It is the ability of the atom to attract the electrons of the chemical bond towards itself. Polar compound? It is covalent compound which the difference between the electronegativity values of their element is relatively high C) Locate the position of the following elements in the modern periodic table: 1- 2He Period 1 group zero 2- 11Na Period 3 group 1A 3- 10Ne Period 2 group zero 4- 16S Period 3 group 6 A 5- 19K Period 4 group 1A 6- 6C Period 2 group 4A 7- 18Ar Period 3 group zero 8- 3Li Period 2 group 1A D) Calculate the atomic number of the following elements: 1-The element in the 2nd period and group (6A). 8 st 2-The element in the 1 period and in group zero. 2 rd 3- The element in the 3 period and group 2A. 12 4-The element in the 3rd period and group (7A). 17 5-The element in the 2nd period and group zero. 10 E) Complete the following statements: 1- The vertical column of elements in the periodic table is called group 2- Elements were arranged in Mendeleev’s table according to atomic weight 3- Elements in the modern periodic table are arranged according to ascending atomic number 4- The modern periodic table consists of 7 periods & 16 groups. 5- Elements of s-block are located on the left side of the periodic table. 6- Elements of p-block are located on the right side of the periodic table. 7- Elements of (B) groups are called transition elements and they appear starting from period 4 F) Give reasons for the following: 1-Both lithium (3Li) and nitrogen (7N) are located in the same period. Because they have the same number of energy levels (2) G) Locate the position of the following elements in the modern periodic table: Element No. of period No. of group Atomic no. symbol Sulphur 16S 3 6A Oxygen 8O 6A Neon 10Ne Potassium 19K Calcium 20Ca 2 2 4 4 Zero 1A 2A