outline_chem2_10-11 - IDS
advertisement

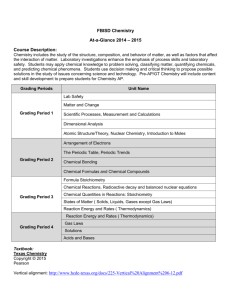
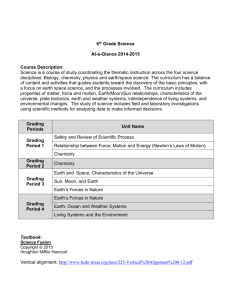
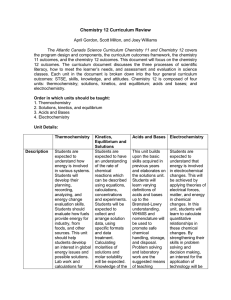
INTEGRATED DEVELOPMENTAL SCHOOL SCHOOL YEAR 2010-2011 SCIENCE AND MATHEMATICS DEPARTMENT COURSE OUTLINE CHEMISTRY 2 General Chemistry 2 First Grading I. Stoichiometry A. The Mole & Chemical Quantities B. Calculation 1. Percentage Composition 2. Empirical Formula 3. Molecular Formula C. Interpretation of Chemical Equation D. Quantitative Info from Balanced Equation E. Limiting Reagent/Excess Reagent F. Percentage Yield II. Solutions A. Nature of Solutions B. Types of Solutions & Solubility C. Concentrations of Solutions 1. Qualitative 2. Quantitative D. Dilution E. Solubility 1. Factors Affecting Solubility 2. Saturated, Unsaturated, Supersaturated F. Colligative Properties G. Colloid 1. Classification & Properties 2. Preparation & Breaking of Colloids Second Grading III. Chemical Kinetics A. Reaction Rates B. Collision Theory C. Factors Affecting Reaction Rates D. Reaction Mechanism & Rate Law 1. Determination of Rate Expressions, Rate Constant, Order of Reaction IV. Chemical Equilibrium A. Systems in Equilibrium B. Determination of Equilibrium-Constant Expression C. Factors Affecting Equilibrium D. Application of Kc/Q E. Le Chatelier’s Principle TEXTBOOK REFERENCES Third Grading V. Acids and Bases A. Properties of Acids and Bases B. Acid-Base Theories C. Strength of Acids and Bases D. Ionization of Water E. pH and pOH and Neutralization F. Indicators and Buffers G. Buffers & Common-Ion Effect H. Titration VI. Electrochemistry A. Oxidation Number & REDOX Reactions B. Balancing Redox Reaction C. Electrolytic Cell & Chemical Potential D. Voltaic/Galvanic Cell E. Practical Cells F. Electrolysis Fourth Grading VII. Thermochemistry A. Thermochemistry vs Thermodynamics B. Internal Energy, Heat, and Work C. The First Law of Thermodynamics D. Enthalpy E. The Spontaneity of Chemical and Physical Changes F. Calorimetry G. Hess’s Law H. Standard Heats of Reaction I. Entropy and Entropy Changes J. The Second Law of Thermodynamics K. The Third Law of Thermodynamics VIII. Nuclear Chemistry A. Terms and Symbols Used B. Radioactivity C. Types of Radioactive Emission D. Half-life E. Transmutation F. Writing and Balancing Nuclear Reactions G. Mass Defect & Binding Energy H. Nuclear Fission & Fusion I. Nuclear Reactors J. Uses and Effects of Radioisotopes Breaking Through Chemistry by Saranay M. Baguio 2006 Conceptual & Functional Chemistry Modular Approach by Padolina, Cristina D, PhD, et. al. 2004 General Chemistry: Principles & Modern Applications, 8 th ed by Petrucci, R., W. Harwood & G. Herring Chemistry: The Central Science 8th ed by Theodore Brown, Eugene LeMay & Bruce Bursten. 2000 Chemistry by E, Mendoza & T. Religioso. Phoenix Publishing House, Inc. Quezon City. 1995