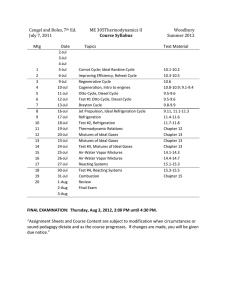
2000-2001 Catalog Data:
advertisement

ENGR 312- Thermodynamics 2001-2002 Catalog Data: Prerequisites by Topic: Textbook: ENGR 312. (4 credits). Applications: Machine and cycle processes, thermodynamic relations, non-reactive gas mixtures, reactive mixtures, thermodynamics of compressible fluid flow. Must be taken in order. PREREQ: MTH 256; CH202 for ENGR 311. Lec/rec. 1. Engineering: Thermodynamics (ENGR 311). 2. Math: Calculus of two-variable functions. 3. Computer: Use of computer to read thermo properties. Moran, M. J., Shapiro, H. N., Fundamentals of Engineering Thermodynamics, John Wiley & Sons, Inc., New York, NY, second edition, 1992. Course Learning Objectives: By the completion of this course, students are expected to... 1. 2. 3. 4. 5. Use the laws of Thermodynamics to analyze and design basic power and refrigeration cycles. Describe the origin of thermodynamic properties and the building of property tables for any simple compressible substance. Apply fundamentals of thermodynamics to problems involving either reacting (e.g. combustion) or non-reacting (e.g. psychrometry) gas mixtures. Determine the equilibrium composition of a reacting mixture. Calculate characteristics of subsonic and supersonic flow through nozzles and ducts with account for a normal shock if present in the flow. Topics: 1. 2. 3. 4. 5. 6. Vapor and gas power cycles (2 weeks) Vapor compression refrigeration (1 week) Introduction to compressible flow. Isentropic flow. Normal shock front. (2 weeks) Maxwell relations. Born-Tisza square. Clapeyron equation of state. Enthalpy, internal energy and entropy differentials in terms of P, v and T differentials and of cp, (cv). Law of corresponding states. Reduced enthalpy and entropy relations and charts (2 weeks) Nonreactive mixtures. Introduction to psychrometrics. Mass and energy conservation in moist air processes. Adiabatic saturation. Wetbulb temperature. Dew point. Psychrometric chart. (1 week) Reactive mixtures. Combustion. Stoichiometry. Energy conservation. Standard enthalpy of combustion of a fuel. Adiabatic flame temperature. Chemical equilibrium. Equilibrium constant. Equilibrium composition calculation. Equilibrium flame temperature and product composition. (1 week) Schedule: Lecture: 1 hour three times per week Recitation: 2 hours once per week. Prepared by A. M. Kanury Date: May 2002 Ability to design and conduct experiments, as well as to analyze and interpret data. Ability to design a system, component, or process to meet desired needs. Ability to function on multidisciplinary teams. Ability to identify, formulate, and solve engineering problems. Understanding of professional and ethical responsibility. Ability to communicate effectively. Broad education necessary to understand the impact of engineering solutions in a global and societal context. Recognition of the need for, and an ability to engage in, life-long learning. Knowledge of contemporary issues. Ability to use the techniques, skills, and modern engineering tools necessary for engineering practice. Ability to apply advanced mathematics through multivariate calculus and differential equations. Familiarity with statistics and linear algebra. Knowledge of chemistry and calculus-based physics with depth in at least one. Ability to work professionally in the thermal systems area including the design and realization of such systems. Ability to work professionally in the mechanical systems area including the design and realization of such systems. ABET Requirements Course Learning Objectives (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) (n) (o) (p) Objective 1 Objective 2 Objective 3 Objective 4 Objective 5 S S S L L P P S S S S S P P P P P P L P P SUMMARY S P P S P Prepared by A. M. Kanury S S L S S P S S S = Substantial correspondence L = Limited correspondence P = Potential for correspondence (instructor dependent) Date: May 2002 Student Self Assessment of Capability Ability to apply math, science, and engineering. ENGR 312- Thermodynamics Course Learning Objectives Mapped to ABET Goals