doc
advertisement
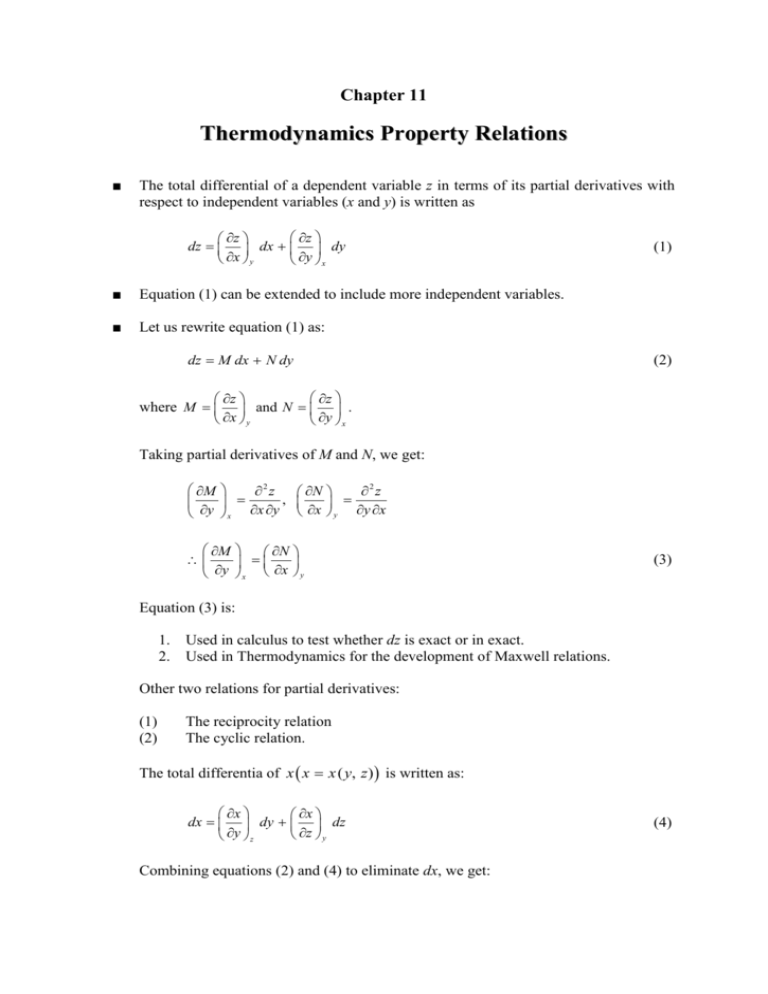
Chapter 11 Thermodynamics Property Relations ■ The total differential of a dependent variable z in terms of its partial derivatives with respect to independent variables (x and y) is written as z z dz dx dy x y y x ■ Equation (1) can be extended to include more independent variables. ■ Let us rewrite equation (1) as: dz M dx N dy (1) (2) z z where M and N . x y y x Taking partial derivatives of M and N, we get: M 2 z N 2 z , y x x y x y y x M N y x x y (3) Equation (3) is: 1. 2. Used in calculus to test whether dz is exact or in exact. Used in Thermodynamics for the development of Maxwell relations. Other two relations for partial derivatives: (1) (2) The reciprocity relation The cyclic relation. The total differentia of x x x ( y, z) is written as: x x dx dy dz z y y z Combining equations (2) and (4) to eliminate dx, we get: (4) 2 z x z x z dz dy dz z y x y x y y z x x Rearranging we get: z x z x z dy 1 dz z x x y x y y z y x (5) For equation (5) to be valid, the terms in brackets must be equal to zero. Hence, we get: 1 x z z y x Reciprocity relation y and x y z 1 y z z z x y Cyclic relation The cyclic relation is frequently used in Thermodynamics. THE MAXWELL RELATIONS ■ Maxwell relations are equations that relate the partial derivatives of P, v, t and s of a simple compressible system. ■ Recall the following T-s (Gibbs) relations: du Tds Pdv (6) dh Tds vdP (7) Two combination properties are given as: a u Ts (Helmholtz function) (8) g h Ts (Gibbs function) (9) Differentiating equations (8) and (9) and using equations (6) and (7), we get two more Gibbs relations: da sdt Pdv (10) dg sdT vdP (11) Gibbs relations (equations (6), (7), (10), and (11)) are of the form: 3 dz M dx N dy Since u, h, a, and g are properties, then their differentials are exact. Therefore, applying M N y x x y we get: T P v s s (12) T P s s P (13) s P T T (14) s P T T P (15) Equations (12) to (15) are called Maxwell relations. They provide means for calculating change in entropy (cannot be measured) from measured properties (P, T, and ν). Also, they are used to derive useful Thermodynamics relations. See Example 11.4. THE CLAPEYRON EQUATION ■ Consider the following Maxwell relation: P s T T (14) During a phase change process, equation (14) can be integrated to give: dP sg s f g f dT sat (16) or sf g dP dT sat f g (17) Also using equation (7), an expression for the change in enthalpy during the phase change process (P and T are constants) can be obtained as: 4 dh Tds dP 0 Tds or g f g dh T ds f Hence, hf g T s f g (18) Substituting equation (18) into equation (17), we get: hf g dP dT sat T f g (19) Equation (19) is known as Clapeyron equation. It can be used to determine the enthalpy change (hfg) from the knowledge of P, ν, and T. See Examples 11.5 and 11.6. GENERAL RELATIONS FOR du, dh, ds, AND SPECIFIC HEATS ■ Internal energy changes: u u (T , ) Hence, the total differential of a is written as: u u du dT du T T Using Gibbs and Maxwell relations, the change in internal energy for a simple compressible substance is given as: T2 u2 u1 Cv dT T1 2 1 P T P du T (20) and similarly ● The change in enthalpy is given as: T2 h2 h1 C p dT T1 ● P2 P1 dP T T P The change in entropy is given as: (21) 5 s2 s1 P2 dT dP P1 T T P Cp T2 T1 ● (22) The change in specific heats is given as: C p Cv T 2 (23) where the volume expansivity β is given as: 1 T P and the isothermal compressibility α is given as: 1 P T See Examples 11.7 to 11.9. THE JOULE-THOMSON COEFFICIENT ■ The Joule-Thomson coefficient is defined as a measure of change in temperature with pressure during a constant-enthalpy process, written as T P h J T ■ (24) 0 temperature increases 0 temperature remains constant 0 temperature decreases The Joule-Thomson coefficient J T in terms of Cp is written as: J T 1 Cp T T T P P h (25)