Matter Unit Test Review
advertisement

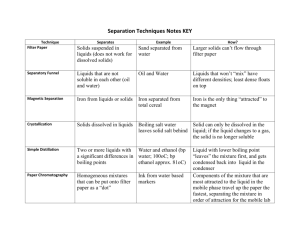
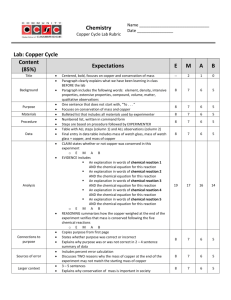
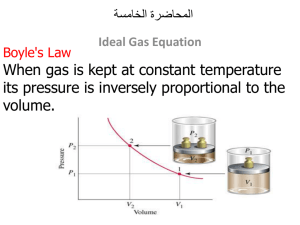
Matter Unit Test Review Define each of the following terms: matter solid liquid gas mass volume physical property/change chemical property/change law of conservation of mass Concepts: know the particle arrangement and movement in solids, liquids, and gases identify characteristics of pure substances identify physical and chemical properties identify physical and chemical changes Read Graphs Practice Problems: 1. A solid has a _______________ shape & ______ ________ volume. 2. A liquid has an______ _____ shape & ________ ________ volume. 3. A gas has an _______ _____ shape & _________ _____ volume. 4. Describe the arrangement of particles in solids, liquids, and gases. 5. Which state of matter is the densest? Which is most easily compressed? 6. Which of the properties below is/are physical properties, and which is/are chemical properties? a. Oxygen atoms can combine with hydrogen atoms to form water molecules. b. Ethyl alcohol boils at 78°C. c. Liquid oxygen is pale blue in color. 7. Which of the changes below are physical changes, and which are chemical changes? a. A copper strip is hammered flat to make a bracelet. b. Copper and sulfur react to form a new substance, copper (I) sulfide. c. Liquid water freezes at 0°C. d. Oxygen gas condenses to a liquid at -183°C. e. You prepare scrambled eggs for breakfast. 8. Which of the symbols below represent elements, and which represent compounds? a. S b. H2O c. C d. N2O5 e. NaOH 9. Is each of the properties below a physical or chemical property? a. temperature at which a solid is converted to a liquid b. odor c. temperature at which a compound breaks down into its elements d. oxygen reacts with a substance to produce energy 10. Distinguish between an element and a compound. 11. Classify the following as being primarily a physical or primarily a chemical change: a. formation of a snowflake b. freezing ice cream c. boiling water d. churning cream to make butter e. boiling an egg f. souring milk 12. How are Elements arranged on the periodic table? 13. Give an example of a reaction: listing reactants and products 14. For the following element: -# of Protons ____ -# of Neutrons ____ -# of Electrons _____ 9 F 19.0 15. What are the charges: a. Electrons ______ b. Protons ______ c. Neutrons ______