Yeast and fermentation: the best kind of sugar Yara Veenstra
advertisement

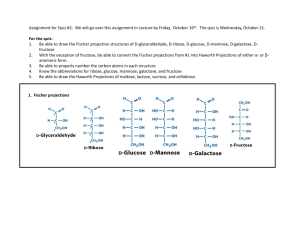
Yeast and fermentation: the best kind of sugar Yara Veenstra & Caroline Vuong 12 April 2010 Summary Because the fossil fuels run out we have to find new fuel sources, for instance you can use ethanol. Ethanol, together with carbon dioxide, is produced by fermenting sugar with yeast. This process is called fermentation. Fermentation occurs in an oxygen free environment. In the fermentation process you can use different kinds of sugar. We made an inquiry where we tried to find the way to get as much ethanol as possible by using different kinds of sugar. We fermented sucrose with baker’s yeast and fructose with baker’s yeast. We found out that sucrose produces a greater amount of carbon dioxide than fructose. Introduction Ethanol fermentation is a biological process in which sugars are converted into cellular energy and carbon dioxide and ethanol. This process is for example responsible for the rising of bread dough. There’s no alcohol in baked bread, because all the ethanol evaporated. Ethanol fermentation is also important for wine-making. Yeast cells are living organisms who convert sugar into carbon dioxide and ethanol. They use sugars as source of energy, so they don’t need the sun to grow. Yeasts are eukaryotes and classified in the kingdom of Fungi. Saccharomyces cerevisiae cells are known as baker’s yeast or brewer’s yeast. In the alcohol industry these organisms have been used to ferment the sugars of rice, wheat and corn to produce alcoholic beverages. In the baking industry they’ve been used to expand or raise dough. Commonly it’s used as baker’s yeast and for some types of fermentation. In this experiment we use S. cerevisiae cells. Those cells use three major pathways to grow. The first pathway is the fermentation of glucose: C6H12O6 (s) 2CH3CH2OH (l) + 2CO2 (g) Second, the oxidation of glucose: C6H12O6 (s) + 602 (g) 6CO2(g) + 6H2O(l) Third, the oxidation of ethanol: CH3CH2OH(l) + 3O2(g) 2CO2(g) + 3H2O(l) If you look at these three pathways, you can see that S. cerevisiae cells can grow in an oxygen free and in an oxygen rich environment. The first pathway is the most interesting for our inquiry, because that involves the production of ethanol and carbon dioxide. Sucrose, commonly known as table sugar, is a disaccharide which first must be converted to fermentable sugars . In figure 1 is sucrose hydrolyzed to glucose and fructose by invertase, an enzyme in yeast. There’re different kind of sugars: sucrose, Dfructose etc. This raises the question: with what kind of sugar (sucrose or fructose) is the amount of produced ethanol the highest in an oxygen free environment? Our hypothesis is that with sucrose the amount of produced ethanol is the highest, because with sucrose the yeast cells have to convert glucose and fructose. Sucrose has to be hydrolyzed first. By using fructose the yeasts only have to convert fructose. Figure 1: Sucrose hydrolyzed to glucose and fructose Experimental design Before conducting the experiments we calculated how much CO2 gas would be produced. We assumed that both experiments would have produced 80 ml of CO2. We put the fructose solution in a flask of 1 liter, we weighted the fructose with a scale to 3 significant digits. Then we divided the fructose solution in three bottles of 250 ml. That way the contents were the same and the results more accurate. During the sucrose experiment we made three separate solutions, we weighted the sucrose with a accurate scale to 4 significant digits. Then one gram baker’s yeast was added to each bottle. The yeast was weighted with the scale to 4 significant digits. To keep the temperature the same while conducting the experiment we placed the bottles in a tank with water at 35°C. We used a gas measurement instrument to measure the amount of produced carbon dioxide. To keep the environment factors the same we conducted the two experiments at the same location. Also we did each experiment in triplicate. That way we can be sure that the measurements are reliable. We assume that 80 ml of CO2 will be formed. the amount of gas didn’t rise anymore. The results of the experiment is presented in table 1. On another day we did the fructose experiment. The results of this experiment is presented in table 2. Sucrose experiment Number Amount of of bottle sucrose in grams per 250 ml Amount of used yeast in grams Produced carbon dioxide in ml Bottle 1 Bottle 2 Bottle 3 1,024 1,032 1,036 80 77 86 1,005 1,058 1,080 Table 1: Produced carbon dioxide (in ml) using sucrose Fructose experiment Number Amount of of bottle sucrose in grams per 250 ml Amount of used yeast in grams Produced carbon dioxide in ml Bottle 1 Bottle 2 Bottle 3 1,020 1,008 1,004 62,5 60 62,5 0,28 0,28 0,28 Table 2: Produced carbon dioxide (in ml) using fructose Data analysis The temperature of the gas measurement instrument was at room temperature. That’s why we used the molar volume at 25ºC = 298 K. The molar volume = 2,45·10-2 m3 mol-1 = 24,5 L ·mol-1 = 24,5·103 mL ·mol-1. Results The experiment took two days. First we did the sucrose experiment. Within a day we observed that the amount of produced carbon dioxide started to rise. At the end of the day By using sucrose is: - 80 mL carbon dioxide equal to 80mL/24,5·103 mL ·mol-1 = 3,3·10-3 mol CO2; - 77 mL carbon dioxide equal to 77mL/24,5·103 mL ·mol-1 = 3,1·10-3 mol CO2; - 86 mL carbon dioxide equal to 86mL/24,5·103 mL ·mol-1 = 3,5·10-3 mol CO2; The average maximum amount of produced CO2 is 3,3·10-3 mol CO2. The formula of the fermentation of glucose: C6H12O6 (s) 2CH3CH2OH (l) + 2CO2 (g) The mol ratio 2CH3CH2OH : 2CO2= 2:2 = 1:1 So there’s 3,3·10-3 mol CH3CH2OH formed by using sucrose. By using fructose is: - 62,5mL carbon dioxide equal to 62,5mL/24,5·103 mL·mol-1 = 2,6·10-3 mol CO2; - 60,0mL carbon dioxide equal to 60,0mL/24,5·103 mL·mol-1 = 2,4·10-3 mol CO2; - 62,5mL carbon dioxide equal to 62,5mL/24,5·103 mL·mol-1 = 2,6·10-3 mol CO2; The average maximum amount of produced CO2 is here 2,5·10-3 mol CO2. So there’s 2,5·10-3 mol CH3CH2OH formed by using fructose. Conclusion and discussion By using sucrose there is more carbon dioxide produced than by using fructose. If the amount of carbon dioxide is higher than the amount of ethanol is also higher. This concludes that you get the highest amount of ethanol by using sucrose in the fermentation process. The average of the produced carbon dioxide using sucrose is 81 ml. That’s more than we expected. However the amount of produced ethanol using fructose is less than 80 ml. For the production of ethanol, using sucrose is more effective than using fructose. This conclusion raises the question what other variables are having an influence at the produced ethanol. Evaluation First we did an inquiry in which we tried to find the optimal pH-values, however the experiment completely failed. We used balloons to measure the amount of CO2, but after a while the balloons started to shrink. The cause for the shrinking was probably the heat of the bottles, which were kept at 35 °C. After the failed experiment we started another inquiry and used a different way of measuring the amount of CO2. This time we used three gas measurement instruments instead of the balloons. The gas measurement instrument are much more reliable and accurate since the CO2 is unable to get out of the gas measurement instrument. The temperature of the bottles was different from the temperature of the gas measurement instruments. The temperature of the bottles was kept at 35°C while the gas measurement instruments were at room temperature. This might have influenced the results. Also since the room temperature wasn’t exactly the same when conducting the experiments, the results might have been affected. When weighing the fructose we used a less accurate scale than for weighing the sucrose. This may have lead to a less accurate measure of fructose, which may have had influence on the results. However because we filled the three bottles of the fructose experiment with the same fructose solution the concentration fructose was exactly the same. So it was more accurate than the sucrose experiment in which we put the sugar individually in the three bottles. Also the yeast was put individually in all of the bottles, so not every bottle had the exact same amount of yeast. The two experiments weren’t conducted at the same time. That was due to the gas measurement instruments. We had just three gas measurement instruments and couldn’t do all the experiments at the same time. So there might have been a difference in the environmental factors which might have affected the measurements. We turned the gas measurement instrument off when we thought there would be no more produced CO2. This might have resulted in a less amount of CO2 then what would have been produced if the gas measurement instrument was turned on for a longer time. This concludes that our inquiry still has much room for improvement. Bibliography 1. BINAS havo/vwo, vijfde druk. 2. Practical Assignment Chemistry, Fermentation 3. http://www.microbiologybytes.com/vi deo/Scerevisiae.html 4. http://www.yeastgenome.org/VLwhat_are_yeast.html 5. www.sigmaaldrich.com/.../enzymatickits.html ICD9_Yara_Caroline