Local Coverage Article for ICD-9 for Anti-Cancer
Drugs - Oxaliplatin (A45216)
Contractor Information
Contractor Name
Pinnacle Business
Solutions, Inc. Arkansas
Back to Top
Article Information
General Information
Article ID Number
A45216
Article Type
Article
Key Article
Yes
Article Title
ICD-9 for Anti-Cancer Drugs Oxaliplatin
AMA CPT / ADA CDT Copyright
Statement
CPT codes, descriptions and other
data only are copyright 2011
American Medical Association (or
such other date of publication of
CPT). All Rights Reserved.
Applicable FARS/DFARS Clauses
Apply. Current Dental
Terminology, (CDT) (including
procedure codes, nomenclature,
descriptors and other data
contained therein) is copyright by
the American Dental Association.
© 2002, 2004 American Dental
Association. All rights reserved.
Applicable FARS/DFARS apply.
Original Article Effective Date
07/15/2007
Article Revision Effective Date
11/01/2011
Article Text
Pinnacle Business Solutions, Inc as a Medicare carrier has determined that the following
anti-cancer drugs may be billed with the specified diagnosis codes only, as of
07/15/2007. Guidelines for coverage of anti-cancer drugs include FDA approval for
specific indications and citation in the USPDI (United States Pharmacopeia Drug
Information) and/or AHFS (American Hospital Formulary Service Drug Information)
providing support for the drug. Text analysis determines the support of a particular use.
Please refer to the Medicare Benefit Manual (Pub.100-02) Chapter 15, Section 50.4.5 for
additional information regarding indications and limitations of coverage and/or medical
necessity as well as documentation requirements.
This is notification that effective July 15, 2007, the following HCPCS codes and
associated ICD-9 codes will be placed on an active audit for verification of appropriate
drug/diagnosis. Claims for anti-cancer drugs billed without a specified allowable
diagnosis will be denied. Approved ICD-9 codes will be updated to reflect changes in
indications and approval as noted by the FDA, AFHS, and/or USPDI.
Back to Top
Coding Information
CPT/HCPCS Codes
J9263
INJECTION, OXALIPLATIN, 0.5 MG
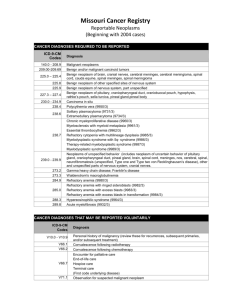
ICD-9 Codes that are Covered
150.0 150.9
151.0 151.9
153.0 153.9
154.0 154.8
155.1
156.0
156.1
156.2
156.8
156.9
157.0 157.3
157.8
157.9
158.8
MALIGNANT NEOPLASM OF CERVICAL ESOPHAGUS MALIGNANT NEOPLASM OF ESOPHAGUS UNSPECIFIED
SITE
MALIGNANT NEOPLASM OF CARDIA - MALIGNANT
NEOPLASM OF STOMACH UNSPECIFIED SITE
MALIGNANT NEOPLASM OF HEPATIC FLEXURE MALIGNANT NEOPLASM OF COLON UNSPECIFIED SITE
MALIGNANT NEOPLASM OF RECTOSIGMOID JUNCTION MALIGNANT NEOPLASM OF OTHER SITES OF RECTUM
RECTOSIGMOID JUNCTION AND ANUS
MALIGNANT NEOPLASM OF INTRAHEPATIC BILE DUCTS
MALIGNANT NEOPLASM OF GALLBLADDER
MALIGNANT NEOPLASM OF EXTRAHEPATIC BILE DUCTS
MALIGNANT NEOPLASM OF AMPULLA OF VATER
MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF
GALLBLADDER AND EXTRAHEPATIC BILE DUCTS
MALIGNANT NEOPLASM OF BILIARY TRACT PART
UNSPECIFIED SITE
MALIGNANT NEOPLASM OF HEAD OF PANCREAS MALIGNANT NEOPLASM OF PANCREATIC DUCT
MALIGNANT NEOPLASM OF OTHER SPECIFIED SITES OF
PANCREAS
MALIGNANT NEOPLASM OF PANCREAS PART
UNSPECIFIED
MALIGNANT NEOPLASM OF SPECIFIED PARTS OF
PERITONEUM
158.9
162.0 162.9
174.0 174.9
175.0
175.9
183.0 183.9
186.0 186.9
197.0
197.6
197.7
200.00 200.88
202.00 202.98
204.10
204.12
235.2
235.5
MALIGNANT NEOPLASM OF PERITONEUM UNSPECIFIED
MALIGNANT NEOPLASM OF TRACHEA - MALIGNANT
NEOPLASM OF BRONCHUS AND LUNG UNSPECIFIED
MALIGNANT NEOPLASM OF NIPPLE AND AREOLA OF
FEMALE BREAST - MALIGNANT NEOPLASM OF BREAST
(FEMALE) UNSPECIFIED SITE
MALIGNANT NEOPLASM OF NIPPLE AND AREOLA OF
MALE BREAST
MALIGNANT NEOPLASM OF OTHER AND UNSPECIFIED
SITES OF MALE BREAST
MALIGNANT NEOPLASM OF OVARY - MALIGNANT
NEOPLASM OF UTERINE ADNEXA UNSPECIFIED SITE
MALIGNANT NEOPLASM OF UNDESCENDED TESTIS MALIGNANT NEOPLASM OF OTHER AND UNSPECIFIED
TESTIS
SECONDARY MALIGNANT NEOPLASM OF LUNG
SECONDARY MALIGNANT NEOPLASM OF
RETROPERITONEUM AND PERITONEUM
MALIGNANT NEOPLASM OF LIVER SECONDARY
RETICULOSARCOMA UNSPECIFIED SITE - OTHER
NAMED VARIANTS OF LYMPHOSARCOMA AND
RETICULOSARCOMA INVOLVING LYMPH NODES OF
MULTIPLE SITES
NODULAR LYMPHOMA UNSPECIFIED SITE - OTHER AND
UNSPECIFIED MALIGNANT NEOPLASMS OF LYMPHOID
AND HISTIOCYTIC TISSUE INVOLVING LYMPH NODES
OF MULTIPLE SITES
CHRONIC LYMPHOID LEUKEMIA, WITHOUT MENTION OF
HAVING ACHIEVED REMISSION
CHRONIC LYMPHOID LEUKEMIA, IN RELAPSE
NEOPLASM OF UNCERTAIN BEHAVIOR OF STOMACH
INTESTINES AND RECTUM
NEOPLASM OF UNCERTAIN BEHAVIOR OF OTHER AND
UNSPECIFIED DIGESTIVE ORGANS
Back to Top
Other Information
Other Comments
05/01/2009 - In accordance with Section 911 of the Medicare Modernization Act of 2003,
FI and Carrier Pinnacle Business Solutions, Inc. (Carrier 00524, FI 00021) were
removed from this Article as the claims processing for the state of Rhode Island was
transitioned to MAC - Part A OR B, NHIC.
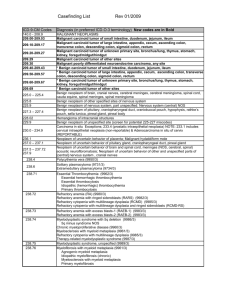
Revision History Explanation
05/31/2007 - Revised to allow effective 07/15/2007.
02/25/2008 - PBSI article retired effective 02/29/08 for New Mexico and Oklahoma
(00521 & 00522) due to the transition of workload to J4 MAC contractor (Trailblazer
Health Enterprises, LLC).
4/11/08 - Added covered ICD-9 Codes 150.0-150.9, 174.0-174.9, 175.0, 175.9, 183.0,
186.0-186.9, 200.00-200.88 & 202.00-202.98 per January 2008 update.
05/28/2008 - PBSI article retired effective 05/31/08 for Missouri (00523) due to the
transition of workload to J5 MAC contractor (Wisconsin Physicians Service).
06/26/2008 - Per NCCN update, expanded covered ICD-9 code 183.0 to range of codes
183.0-183.9 effective 06/05/2008. Also added covered ICD-9 codes 157.0-157.3, 157.8
and 157.9 effective 06/05/2008.
07/18/2008 - Effective retroactively to 07/15/2007, updated covered ICD-9 Codes to
include 162.0-162.9.
05/01/2009 - In accordance with Section 911 of the Medicare Modernization Act of 2003,
FI and Carrier Pinnacle Business Solutions, Inc. (Carrier 00524, FI 00021) were
removed from this Article as the claims processing for the state of Rhode Island was
transitioned to MAC - Part A OR B, NHIC.
10/29/2009 - Per October 2009 update, added covered ICD-9 codes 155.1, 156.0,
156.1, 156.2, 156.8, 156.9 and 235.5 effective 10/01/2009.
11/17/2009 - Amended Article Text to reference National Coverage Determination (NCD)
Pub. 100-02 in place of Local Coverage Determination (LCD) AC-01-024 which has
been retired.
01/20/2010 - Per January 2010 update, added covered ICD-9 codes 158.8, 197.0 and
197.7 effective 01/01/2010.
02/12/2010 - Per February 2010 update, added covered ICD-9 code 235.2 effective
02/01/2010.
04/22/2010 - Per April 2010 update, added covered ICD-9 Codes 197.6, 204.10 and
204.12 effective 04/01/2010.
11/20/2011 - Per November 2011 upodate, added covered ICD-9 Code 158.9 effective
11/01/2011.
Back to Top
All Versions
Updated on 01/24/2012 with effective dates 11/01/2011 - N/A
Updated on 04/22/2010 with effective dates 04/01/2010 - N/A
Updated on 02/12/2010 with effective dates 02/01/2010 - N/A
Updated on 01/20/2010 with effective dates 01/01/2010 - N/A
Some older versions have been archived. Please visit MCD Archive Site to retrieve
them.
Read the Article Disclaimer
Back to Top
Footer Links
Get Help with File Formats and Plug-Ins
•
•
•
Submit Feedback
Department of Health & Human Services
• Medicare.gov
• USA.gov
•
Web Policies & Important Links
• Privacy Policy
• Freedom of Information Act
• No Fear Act
Centers for Medicare & Medicaid Services, 7500 Security Boulevard Baltimore, MD 21244
64