naming packet answers
advertisement
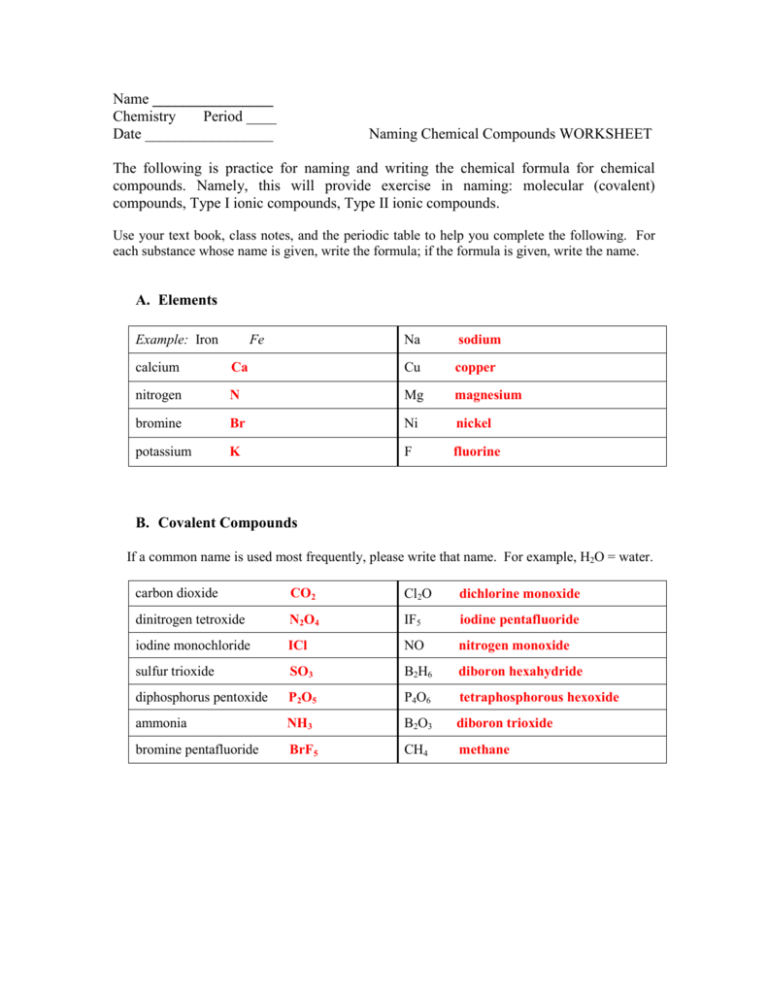
Name ________________ Chemistry Period ____ Date _________________ Naming Chemical Compounds WORKSHEET The following is practice for naming and writing the chemical formula for chemical compounds. Namely, this will provide exercise in naming: molecular (covalent) compounds, Type I ionic compounds, Type II ionic compounds. Use your text book, class notes, and the periodic table to help you complete the following. For each substance whose name is given, write the formula; if the formula is given, write the name. A. Elements Example: Iron Fe Na sodium calcium Ca Cu copper nitrogen N Mg magnesium bromine Br Ni nickel potassium K F fluorine B. Covalent Compounds If a common name is used most frequently, please write that name. For example, H2O = water. carbon dioxide CO2 Cl2O dichlorine monoxide dinitrogen tetroxide N2O4 IF5 iodine pentafluoride iodine monochloride ICl NO nitrogen monoxide sulfur trioxide SO3 B2H6 diboron hexahydride diphosphorus pentoxide P2O5 P4O6 tetraphosphorous hexoxide ammonia NH3 B2O3 diboron trioxide bromine pentafluoride BrF5 CH4 methane C. Type I Ionic Compounds magnesium iodide MgI2 K3P potassium phosphide silver oxide Ag2O Al2O3 aluminum oxide aluminum chloride AlCl3 Ba3N2 barium nitride calcium oxide CaO Na2S sodium sulfide potassium sulfide K2S LiF lithium fluoride cesium fluoride CsF CaI2 calcium iodide strontium bromide SrBr2 ZnCl2 zinc chloride D. Type II Ionic Compounds lead (II) oxide PbO Cu2O copper (I) oxide tin (II) chloride SnCl2 CrCl2 chromiun (II) chloride manganese (IV) oxide MnO2 SnCl4 tin (IV) chloride vanadium (V) oxide V2O5 PtF4 platinum (IV) fluoride lead (IV) iodide PbI4 OsO2 osmium (IV) oxide nickel (III) nitride NiN Fe2O3 iron (III) oxide E. Ionic Compounds (Type I & Type II) with POLYATOMIC IONS sodium hydroxide NaOH Al2(SO4)3 aluminum sulfate tin (IV) carbonate Sn(CO3)2 Fe(OH)2 iron (II) hydroxide barium nitrate Ba(NO3)2 (NH4)2CO3 ammonium carbonate ammonium acetate NH4C2H3O2 Ca(ClO3)2 calcium chlorate iron (III) nitrate Fe(NO3)3 Pb(C2H3O2)4 lead (IV) acetate aluminum nitrite Al(NO2)3 K3PO4 potassium phosphate cobalt (III) sulfate Co2(SO4)3 Mg(NO2)2 magnesium nitrite F. Putting it all together Given sodium sulfide potassium phosphate xenon hexafluoride Covalent, Ionic I or Ionic II? Ionic I Ionic I Formula / Name Na2S K3PO4 Covalent XeF6 Ionic II Fe2O3 Covalent N2O4 Ionic II SnO2 boron trichloride Covalent BCl3 aluminum sulfate Ionic I Al2(SO4)3 cobalt (II) sulfide Ionic II CoS ammonium phosphide Ionic I (NH4)3P iron (III) oxide dinitrogen tetroxide tin (IV) oxide tetracarbon decahydride Covalent C4H10 sodium hydroxide Ionic I NaOH copper (II) bromide Ionic II CuBr2 iodine monochloride Covalent Pb(NO3)2 ICl Ionic II lead (II) nitrate AsI3 Covalent arsenic triiodide MgI2 Ionic I B2O3 Covalent magnesium iodide diboron trioxide (NH4)2CO3 Ionic I ammonium carbonate Co2S3 Ionic II cobalt (III) sulfide P2Cl4 Covalent diphosphorous tetrachloride BF3 Covalent boron trifluoride CsBr Ionic I cesium bromide CaCO3 Ionic I calcium carbonate CuO Ionic II copper (II) oxide BeO Covalent beryillium oxide H2O Covalent water G. Some questions for you: 1. How does one identify if a compound is a molecular (covalent) compound? Non-metal+ non-metal 2. How does one identify if a compound is an ionic compound? Non-metal + metal 3. How do you know when to use Roman Numerals? Why do you use them? Use roman numerals in type II ionic compounds. They are used to identify the charge on type II metals. They are necessary because type II metals can change their charge. 4. Can a compound with a polyatomic ion end with an “ide” (like sodium chloride)? Why or why not? Yes it can, but only if the name already ends in “-ide”, like hydroxide. Do not change the name to end in “-ide” 5. True or False? A compound with a polyatomic ion must contain a parenthesis with a numerical subscript, like (NO3)2. Explain. False; you only need parenthesis, if you have more than one polyatomic ion. 6. True or False? Before naming a molecular (covalent) compound, you must reduce the subscripts to the smallest whole number ratio, for example, Si2H4 SiH2 so the name is silicon dihydride. Explain. False. You only reduce the subscripts for ionic compounds.





