Sand salt ammonium chloride sep. Lab
advertisement

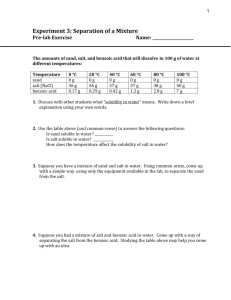
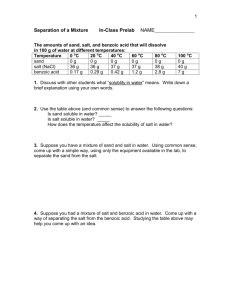
Lab #2: Separation of a Three Component Mixture Physical and chemical properties are very useful when trying to purify substances. Most substances found in nature are present in complex mixtures that require many steps in order to separate the components. The purpose of this lab is to use physical and chemical properties of three substances in order to separate them from each other. Once separated, the initial percentages of each component will be calculated. Equipment - electronic balance - Bunsen burner - ring stand set-up - graduated cylinder - evaporating dish - scoop - 150 mL beaker - filter paper - funnel - stirring rod Pre-Lab Read through the procedure and set up a data table to record the various measurements. This should be a simple two-column table where the first column indicates the measurement to be made and the second column provides a place for the measurement to be written. Be sure to include both error ranges and units when you actually write down the data. Procedure 1. Obtain the mass of a clean 150 mL beaker and record. 2. Obtain between 2 – 3 grams of mixture sample and record its sample number. This mixture contains three components: sand (SiO2), table salt (NaCl), and ammonium chloride (NH4Cl). Obtain the mass and the beaker with the mixture. Record this and then calculate/record the mass of the mixture. 3. Place the beaker and its contents on the ring stand and heat the beaker with a Bunsen burner. Begin heating near the top of the beaker and slowly move the flame toward the bottom. Heat the whole beaker with a double-blue flame until you no longer see white vapors coming off and no white residue left on the top of the baker. The ammonium chloride will be separated from the sand and salt during this process. 4. Allow the beaker to cool to room temperature and then obtain the mass of the cooled beaker and remaining mixture. Record this mass. Now calculate/record the mass of NH4Cl that was removed from the mixture. 5. Add 50 mL of water to the remaining mixture. Stir with the stirring rod for about 3 minutes, making sure to break up any larger chunks of mixture. 6. Obtain the mass of a piece of filter paper. Record the mass and then fold the filter paper for use in a funnel. 7. Swirl the salt solution and undissolved sand in the beaker and pour these onto the filter paper. You may use more water to rinse any remaining solid into the filter paper. 8. Once the beaker is emptied pour an additional 10 mL of water through the filter paper to make sure that all of the salt solution is rinsed through. 9. Remove the filter paper from the funnel and lay it flat to dry overnight. Once dry, record the mass of the filter paper and sand. Then calculate/record the mass of the sand. Conclusions & Analysis 1. Show the calculations for the masses of each component in the mixture including final error ranges for the results. Then calculate the percent composition of each substance in the mixture, again using error ranges along with the results. 2. Identify the physical changes involved in separating these components from the mixture. Based on what you observed during the removal of the ammonium chloride, give one reason for believing that this is a physical rather than chemical change (hint: ammonium chloride is a white solid at room temperature). 3. It is possible to continue this experiment past the collecting of the sand. The solution that went through the filter could also have been collected and used to find the mass of the salt that was dissolved in it. Describe the process that would have been used to accomplish separating the dissolved salt from the water. Be sure to indicate any additional experimental steps and measurements that would have been necessary in order to do this. 4. Explain/Describe what would happen to the percentages of each component in the mixture if the following errors occurred. Be sure to be specific in how the final results for each component percentage would be altered and explain your reasoning. a) The beaker is not completely heated in step #3 and ammonium chloride is left in the mixture beaker (the ammonium chloride will dissolve in water later on). b) A student accidentally added 500 mL of water in step 5 (instead of 50 mL). Scoring Summary Organization write-up neatly presented data table(s) neatly presented w/ units U/A _____ (2) observed lab procedure _____ (2) Precision & Accuracy final results within allowed error _____ (3) proper error ranges for raw data _____ (1) Conclusions & Analysis lab calculations including error (#1) _____ (6) physical changes (#2) _____ (2) procedure description (#3) _____ (5) error analysis (#4) _____ (4)