STRUCTURE OF SYNAPSES: OVERVIEW
advertisement

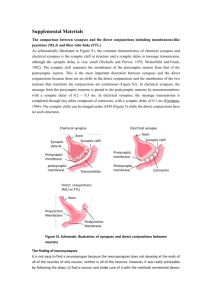
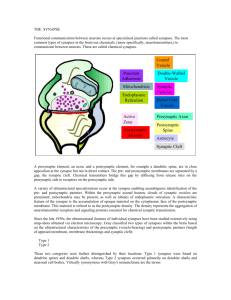
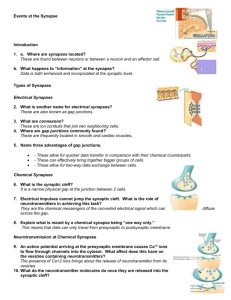
Prof. U.J. McMahan IBRO Lecture STRUCTURE OF SYNAPSES: OVERVIEW Traditionally, a synapse is considered to be a site of apposition between two neurons, where an ion conductance change (impulse) across the plasma membrane of one brings about an ion conductance change across the plasma membrane of the other, in a process all together referred to as synaptic transmission. The term synapse now also applies to functionally similar sites of apposition between receptor cells and neurons, between neurons and muscle fibers and between neurons and gland cells. Because virtually all aspects of animal behavior are dependent on the synaptic transmission of impulses, much of neuroscience is devoted to understanding how synapses function. There are "electrical" synapses, at which there is simply a passive spread of ions from one cell to the other. And there are “chemical” synapses, where the impulse in the presynaptic cell brings about that cell’s release of a chemical neurotransmitter which diffuses across a narrow gap (synaptic cleft) to bind to specific receptors in the plasma membrane of the postsynaptic cell. It is the interaction of the neurotransmitter with its receptors in the postsynaptic plasma membrane that leads to the change in the membrane's conductance. Chemical synapses are the most abundant type in all animal species. Electrical synapses are more abundant in invertebrates than in the vertebrates, and in mammals chemical synapses seem to be the predominant type by far. At both chemical synapses and electrical synapses, there are structural features distinct from those found in other parts of the pre- and postsynaptic cell. These specializations have provided and continue to provide important information as to how the synapses work. This lecture describes prominent specializations together with an overview of their function. ELECTRICAL SYNAPSES When thin sections from from tissues containing electrical synapses are examined by electron microscopy, the plasma membranes of the apposed cells at the synapses are seen to lie much closer to each other than in non-synaptic regions. Indeed the distance separating the bimolecular lipid leaflets of the membranes at these synapses is 2-3nm instead of 15-20nm. Electron microscopy of freeze fracture replicas of the interior of each of the plasma membranes at the synapses show that both contain a hexagonal array of macromolecules. Biochemistry has revealed that the macromolecules are a distinct class of proteins now known as connexons. Each connexon is made up of six subunits, connexins. Moreover, each of the six connexins traverses the plasma membrane, so that it has cytoplasmic and extracellular domains as well as as a membrane domain, and all together they line a transmembrane channel. The connexins of a connexon in one plasma membrane bind to the connexins of a connexon in the adjacent plasma membrane so that the channel of the one is continuous with that of the other. It is these conjoined proteinlined channels between apposed plasma membranes that mediate the passive spread of ions from one cell to the other during electrical transmission. Aggregates of connexons are characteristic of gap junctions, which occur between many types of non-neuronal cells (such as liver cells, where they are involved in the intercellular transfer of ions and small peptides necessary for the maintenance of a tissue’s functional integrity. CHEMICAL SYNAPSES At chemical synapses, there are structural specializations unique for the pre- and postsynaptic cell; those in the presynaptic cell being involved mainly in the storage and release of neurotransmitter, those in the postsynaptic cell being involved in the reception of the neurotransmitter. Moreover, there are specializations in the synaptic cleft involved in maintaining the efficacy of the synapse. Although these specializations are similar for all synapses, there are variations depending on the type of pre- and postsynaptic cell and the animal species. In the following account I describe the structural specializations at common neuron-to-neuron synapses in the vertebrate central nervous system. 1 December, 2003 Prof. U.J. McMahan IBRO Lecture December, 2003 Presynaptic Specializations A neuron has three principal parts based on sturctural and functional differences: a cell body, which contains the nucleus, multiple dendrites and an axon. There are recorded examples of synapses between two neuron cell bodies, between two dendrites, and between two axons. But the most common type of neuron-to-neuron synapse involves an axon as the presynaptic component and a neuron cell body or dendrite as the postsynaptic component. The axonal arborization A neuron is generally innervated by the axons of many neurons. Each of these parent axons extends from the cell body without branching. However, as it approaches the neuron or neurons it innervates, it forms multiple branches. When each of these preterminal branches is within a few microns of the neurons it innervates, it gives rise to an arborization of terminal branches that extend over the neurons’ surface. The number and length of terminal branches varies from one neuron type to another, extending up to many tens of micrometers over a target neuron’s surface. In all cases, however, the terminal branches release transmitter, and the release occurs at multiple sites along the terminal branch. Accordingly, the terminal arborization is an axonal specialization that provides distributed sources of transmitter to the postsynaptic neuron. Varicosities in terminal branches Each terminal branch in the terminal arborization has numerous varicosities (swellings). The varicosities are directly apposed to the postsynaptic neuron. Glial cells can form myelin sheaths on the parent motor axons and their preterminal branches. Terminal branches, on the other hand, are unmyelinated but the processes of glial cells are closely associated with them. While the terminal glial cells can completely wrap around the connectives between varicosities, they are associated only with that portion of the varicosity surface that faces away from the postsynaptic neuron. Indeed there is no intervening cell between the plasma membrane of the varicosity and the plasma membrane of the postsynaptic cell. Although the varicosities directly face the postsynaptic neuron, they are separated from it by a narrow space of ~25 nanometers (nm). Based largely on evidence discussed below (see Active zones) the, neurotransmitter release that occurs during impulse transmission takes place at the varicosities and from that portion of the varicosities that face the postsynaptic neuron. Thus, varicosities provide the terminal branches with transmitter release sites, and these sites are positioned a very short distance from the postsynaptic neuron that is uninterrupted by any other cell, which, altogether, accounts in part for the constant and brief time course between the release of transmitter and the postsynaptic response as recorded electrophysiologically. The portion of the varicosities' plasma membrane that faces the postsynaptic cell is the presynaptic membrane and the 25nm wide space between the presynaptic membrane and the plasma membrane of the postsynaptic neuron (postsynaptic membrane) is the synaptic cleft. Composition of varicosities Each varicosity can be divided into two distict regions: the axon backbone and the varicosity proper. The axon backbone is largely the continuation of axonal components that fill the parent axon, the preterminal axon branches and the connectives between the varicosities. It's main constituents includes mitochondria, lysosomes, microtubules and filaments, and smooth endoplasmic reticulum. In the varicosity proper the most conspicuous components are vesicles and actin filaments. The vesicles fall into several categories according to their form and function. Principal among them are the following: 1) agranular vesicles, that are spherical, ~35-50nm diameter (depending on the synapse), and contain neurotransmitter. These are also known as synaptic vesicles and they are by far the most abundant vesicle in the varicosity proper. They are in clusters that are focused on the presynaptic membrane. They contain the so-called small molecule neurotransmitters that include amino acids (such as glutamate or glycine), catecholamines (such as norepinephrine and dopamine), serotonin or ACh. When the nerve impulse arrives 2 Prof. U.J. McMahan IBRO Lecture at the varicosity, some of the synaptic vesicles fuse with the presynaptic membrane releasing their content into the synaptic cleft to act on receptors specific for the neurotransmitters in the postsynaptic membrane. Thus synaptic transmission relies on the vesicle mediated exocytosis of neurotransmitter. There is usually only one small molecule transmitter for a specific neuron type, but examples of different transmitters being released from the same varicosity have been observed. 2) granular vesicles. These range from 80nm-160nm and they contain an electron dense granule when viewed by conventional electron microscope methods. There are much fewer granular vesicles in a varicosity than synaptic vesicles. The granular vesicles are known to contain peptides, which at many synapses include large molecule neurotransmitters. The peptides are also released from the varicosity by exocytosis. They act on receptors in the postsynaptic neuron to modulate the action of the small molecule neurotransmitters. 3) coated vesicles, cisternae and endosomes. Coated vesicles are about the size of synaptic vesicles and are agranular but their external surface is covered by a network of the protein, clathrin. Cisternae are flattened agranular vesicles of varying dimensions; endosomes are rounded agranular vesicles of varying dimensions, but usually larger than synaptic vesicles. Shortly after fusion of synaptic vesicles with the presynaptic membrane, portions of the membrane are internalized by the varicosity which leads to the local generation new synaptic vesicles loaded with neurotransmitter and available for release during impulse transmission. The coated vesicles, cisternae and endosomes are thought to play a role in the recycling of synaptic vesicle membrane. Actin filaments. The vesicles are not free to move in just any direction within a varicosity. If they were, one might expect to find them uniformly distrubuted. But this is not the case. Rather they are arranged in clusters which are focused on distinct sites on the presynaptic membrane. Among the vesicles are actin filaments. It is not known whether the F-actin is focused on the presynaptic membrane, but there are in varcosities a class of proteins known as synapsins which can cross-link synaptic vesicles to F-actin, inhibiting their movement. The movement of vesicles toward the presynaptic membrane, which must occur as vesicles that have fused with the presynaptic membrane are replaced, may rely on the regulation of interactions between synapsin, synaptic vesicles and actin. Active zones. The sites on the presynaptic membrane at which the vesicle groups are focused are characterized by one or more dense aggregates of proteins that stud the cytoplasmic surface of the memrane. At common neuron-to-neuron synapses the aggregates are more or less conical and they extend about 75nm into the cytoplasm. The base of the cone, about 50 nm diameter, is attached to the presunaptic membrane. The protein aggregates are often in clusters. Synaptic vesicles next to the protein aggregates are docked to (held at) the presynaptic membrane. The presynaptic membrane just beneath each protein aggregates contains aggregates of macromolecules that include voltage dependent calcium channels and calcium activated potassium channels. When a nerve impulse arrives at a varicosity, the depolariztion of the presynaptic membrane causes the calcium channels to open. Calcium that enters the varicosity interacts with proteins that bring about the fusion of docked vesicles with the presynaptic membrane and exocytosis of neurotransmitter. It also interacts with the calcium activated potassium channels, which open to allow the passage of potassium out of the varicosity. This repolarizes the presynaptic membrane, thus closing the calcium channels and terminating the exocytosis of neurotransmitter. The region(s) along the presynaptic membrane of varicosities characterized by one or more protein aggregates, their associated docked vesicles and the subjacent presynaptic membrane with aggregates macromolecules (calcium channels and calcium activated potassium channels) are called axtive zones. It is at the active zones that the initial events in synaptic transmission take place. The aggregates of proteins in the active zones are known as active zone material. The active zone material helps dock the synaptic vesicles and anchor the macromolecules, and it may contain the proteins that mediate the calcium-induced fusion of the docked vesicles with the presynaptic membrane. 3 December, 2003 Prof. U.J. McMahan IBRO Lecture Postsynaptic Specializations Postsynaptic Plasma membrane and subsurface cytoskeleton (Postsynaptic Density ) In the plasma membrane of the postsynaptic cell, just opposite each active zone of the varicosity, there is a high concentration of receptor proteins for the neurotransmitter. At some synapses the concentration of receptors opposite the active zone is more than a thousand times greater than it is anywhere else on the surface of the postsynaptic cell. A dense aggregate of proteins, including cytoskeleton, lines the cytoplasmic surface of the postsynaptic membrane subjacent to the receptor aggregates. It is called the postsynaptic density (PSD). The PSD helps anchor the receptors in the postsynaptic membrane. At certain synapses it also contains proteins that mediate the opening of ion channels in response to the neurotransmitter-receptor interaction. Synaptic Cleft The 25nm wide region of extracellular space between the active zone of the varicosity and the receptor-rich region of the postsynaptic membrane is slightly wider than elsewhere between the pre- and postsynaptic membrane (~15nm). It is referred to as the synaptic cleft. There is little obvious material between neurons and glial cells in the central nervous system and antibodies to extracellular matrix proteins found in abundance in other tissues, such as muscle or kidney, show little to no staining by immunohistochemistry. But at synapses a conspicuously dense aggregate of proteins extends across the synaptic cleft from the pre-to postsynaptic membrane. These proteins do not impede the diffusion of neurotransmitter across the cleft. They include the extracellular portions of receptors and channels and probably proteins involved in maintaining a tight adherence between the preand postsynaptic membrane at active zones. SUMMARY The preceding account describes in brief the structural specializations at electrical and chemical synapses. Those at chemical synapses are more numerous and varied than those at electrical synapses. However, with the exception of synaptic vesicles at chemical synapses, the specializations of both types of synapses consist of aggregates of proteins. At electrical synapses there are aggregates of connexons in the pre and postsynaptic membrane. At chemical synapses there are aggregates of calcium channels and calcium activated potassium channels in the presynaptic membrane, aggregates of receptors for neurotransmitter in the postsynaptic membrane, aggregates of proteins on the cytoplasmic surface of the presynaptic membrane (active zone material), aggregates of proteins on the cytoplasmic surface of the postsynaptic membrane (PSD) and aggregates of proteins in the synaptic cleft. I have pointed out that each specialization plays a specific role in synaptic function. In subsequent lectures I will discuss the evidence for such conclusions as they pertain to the specializations at chemical synapses, and how the specializations are formed and maintained. General References Cowan, M.W., Sudhöf, T.C. and Stevens, C.F. (eds) 2000 Synapses, Johns Hopkins University Press/ Baltimore. Kandel, E.R., Schwartz, J.H. and Jessell, T.M. 1991 Principles of Neuroscience (3rd. ed), Elsevier/New York. Nicholls, J.G., Martin, A.R.,Wallace, B.G. and Fuchs, P.A. 2001 From Neuron to Brain (4th ed), Sinauer Associates, Inc/ Sunderland. 4 December, 2003 Prof. U.J. McMahan IBRO Lecture Peters, A., Palay, S.L. and Webster, H. deF. 1991 The Fine Structure of the Nervous System, Oxford/Oxford. . 5 December, 2003