12:15-1:20PM - Seattle University
advertisement
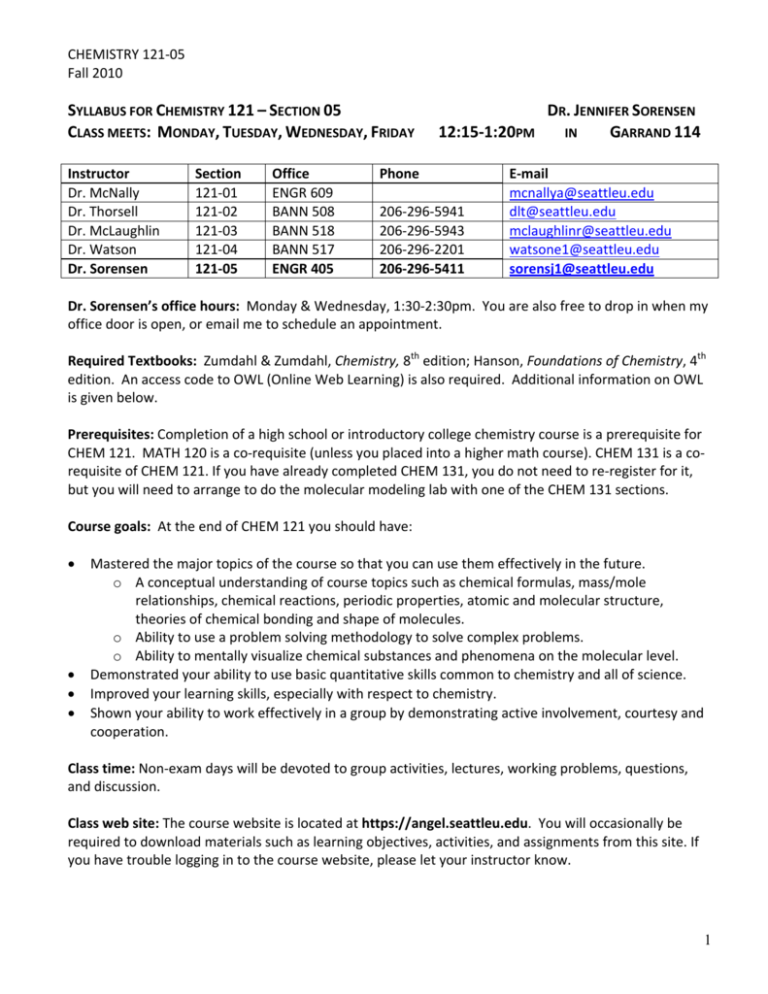
CHEMISTRY 121‐05 Fall 2010 SYLLABUS FOR CHEMISTRY 121 – SECTION 05 CLASS MEETS: MONDAY, TUESDAY, WEDNESDAY, FRIDAY DR. JENNIFER SORENSEN 12:15‐1:20PM IN GARRAND 114 Instructor Section Office Phone E‐mail Dr. McNally 121‐01 ENGR 609 mcnallya@seattleu.edu Dr. Thorsell 121‐02 BANN 508 206‐296‐5941 dlt@seattleu.edu Dr. McLaughlin 121‐03 BANN 518 206‐296‐5943 mclaughlinr@seattleu.edu Dr. Watson 121‐04 BANN 517 206‐296‐2201 watsone1@seattleu.edu Dr. Sorensen 121‐05 ENGR 405 206‐296‐5411 sorensj1@seattleu.edu Dr. Sorensen’s office hours: Monday & Wednesday, 1:30‐2:30pm. You are also free to drop in when my office door is open, or email me to schedule an appointment. Required Textbooks: Zumdahl & Zumdahl, Chemistry, 8th edition; Hanson, Foundations of Chemistry, 4th edition. An access code to OWL (Online Web Learning) is also required. Additional information on OWL is given below. Prerequisites: Completion of a high school or introductory college chemistry course is a prerequisite for CHEM 121. MATH 120 is a co‐requisite (unless you placed into a higher math course). CHEM 131 is a co‐ requisite of CHEM 121. If you have already completed CHEM 131, you do not need to re‐register for it, but you will need to arrange to do the molecular modeling lab with one of the CHEM 131 sections. Course goals: At the end of CHEM 121 you should have: • Mastered the major topics of the course so that you can use them effectively in the future. o A conceptual understanding of course topics such as chemical formulas, mass/mole relationships, chemical reactions, periodic properties, atomic and molecular structure, theories of chemical bonding and shape of molecules. o Ability to use a problem solving methodology to solve complex problems. o Ability to mentally visualize chemical substances and phenomena on the molecular level. • Demonstrated your ability to use basic quantitative skills common to chemistry and all of science. • Improved your learning skills, especially with respect to chemistry. • Shown your ability to work effectively in a group by demonstrating active involvement, courtesy and cooperation. Class time: Non‐exam days will be devoted to group activities, lectures, working problems, questions, and discussion. Class web site: The course website is located at https://angel.seattleu.edu. You will occasionally be required to download materials such as learning objectives, activities, and assignments from this site. If you have trouble logging in to the course website, please let your instructor know. 1 CHEMISTRY 121‐05 Fall 2010 Grading: Your grade in this course is based on 750 points distributed as follows: course exams 300 points final exam 150 points homework and quiz total 160 points formal in‐class activities 100 points molecular modeling lab 40 points (completed during a CHEM 131 session) Course grade: Your total point score will determine your final grade for the quarter. The borderlines between the various grades will be approximately the following percentages of the total points: A to A‐ 100‐87% B+ to B‐ 87‐73% C+ to C‐ 73‐60% D+ to D‐ 60‐50% F<50% We do not grade on the curve in CHEM 121, so there is no need to be competitive with your classmates unless it drives you to work harder and do better. There is no limit to the number of A’s (or F’s) that may be given during a quarter. If you earn an A, you will get one, no matter how well your classmates may do. Therefore, you are encouraged to help each other learn as much as possible. Course exams: Four exams will be given during the quarter on the days specified on the course schedule. A missed exam counts as a zero; no make‐up exams are offered, however your lowest exam grade will be dropped. Each exam is cumulative from the beginning of the quarter but will concentrate on material covered since the last exam. On all exams, you will be expected to apply the material covered and to work problems, not just to reproduce facts from memory. When the graded exam is returned to you, it is strongly recommended that you re‐work all missed questions. Do this on the exam in different color ink or pencil than was used to write or grade the exam, then keep the exam in your course portfolio. TO REPEAT: THERE ARE NO MAKE‐UP EXAMS. Final exam: The final exam is comprehensive over the entire quarter and is a multiple‐choice exam. Students who practice problem‐solving regularly and work diligently on in‐class activities tend to improve their performance on exams throughout the quarter, including the final exam. The final exam will be on Thursday Dec. 9, 10:00‐11:50am in our regular classroom. OWL/Homework: Online Web Learning (OWL) is an online homework and tutorial system that supplements the instruction and practice problems in the Zumdahl textbook. Approximately 5 graded homework problems each week will be due via OWL no later than Friday at 3:00pm. You are free to do further ungraded practice problems and tutorials from OWL’s extensive learning resources. In addition to OWL, you may also have graded homework assignments from handouts or the textbook. To access OWL, purchase an Access Code using one of these two options: 1. Bundled with your textbook: Check the text you purchased for an OWL access code card. An access code should come with all new textbooks. 2. Purchase online: If you purchased a used textbook or have chosen to use an electronic textbook, go to www.cengage.com/owl, choose your course, and then choose Buy an Access Code. 2 CHEMISTRY 121‐05 Fall 2010 After you have your access code, you will use it to register for OWL and choose a login and password. See the registration directions at: http://www.cengage.com/chemistry/discipline_content/owl/support/student_quick_start.pdf If you need assistance during the process, see www.cengage.com/owl. Quizzes: Short quizzes (intended to take about five minutes) will be given throughout the quarter to ensure that you are keeping up with the reading and homework problems. These quizzes will be unannounced, and will thus also serve to give you points for attendance. They will be administered at the beginning of the class hour and make‐up quizzes will not be given. Study guides and learning objectives: A set of study guide questions and practice problems will be given with each chapter; these can be downloaded from Angel. The study guides are not graded, but they are the foundation of your daily chemistry practice. Keep all of your study guides and practice problems in your course portfolio. DO NOT NEGLECT TO DO THE PRACTICE PROBLEMS. Diligently working problems is the single‐most effective way of improving your understanding of the course material and your performance on exams. Only through trying to work problems will you learn what you don’t understand about the material. Formal in‐class group activities: There will be many informal class activities (primarily from the Hanson book), but there will also be five class sessions devoted to “formal” group activities. They will focus on particularly important topics of chemistry that are fundamental to this class and your future classes in the chemistry curriculum. For each of these activities there will be a graded: 1. Individual pre‐activity assignment due at the beginning of the activity class day. Late pre‐ activities will not be accepted. 2. In‐class group‐work session. 3. Individual follow‐up homework assignment, due at the beginning of the next class. Calculators: The calculator needed for the CHEM 121‐122‐123 series must perform standard mathematical operations including exponentials and logarithms, both base‐10 logs and natural logs and must be able to deal with scientific (exponential) notation. Programmable graphing calculators will absolutely not be allowed for exams. Likewise, cell phone calculators are not allowed. We require you have an inexpensive scientific calculator ($10‐12) capable of the functions outlined above for use on exams. You should practice with that calculator when doing homework problems. Portfolio (course binder): It is strongly recommended that you use a three‐ring binder at least 1.5” thick that will hold all your materials for CHEM 121 in one place. This course generates many pieces of paper, so it is in your best interest to have an organized system for keeping track of all of your work. Use labeled tab dividers to separate the various sections. 3 CHEMISTRY 121‐05 Fall 2010 The suggested sections and organization of the portfolio are: 1. Course administration: class schedule, syllabus 2. Class notes: include any note sheets handed out in class 3. Formal activities: include pre‐activity, activity and post‐activity assignments 4. Course work: ORGANIZED BY CHAPTERS – printed study guides, written out objectives, worksheets, practice problems, homework 5. Exams: missed problems re‐worked in different color ink Study groups: The SU Learning Center will sponsor regular study group sessions, each led by advanced chemistry students, and running throughout the quarter. Attendance at these study groups is optional but they provide an opportunity to work with your classmates on problems and practice chemistry in a situation where help will be available. More details and the times of the study group meetings will be available during the second week of class. Note about CHEM 131: CHEM 131 is the laboratory course that accompanies CHEM 121. You will receive a separate schedule and syllabus for your CHEM 131 class and there will be a CHEM 131 website where you will download instructions for the experiments. The class meets once per week and will have a pre‐ laboratory lecture in Bannan 501 at your assigned day and time, and then you will go upstairs to your laboratory room. Disabilities and health issues: If you have, or think you may have, a disability (including an ‘invisible disability’ such as a learning disability, a chronic health problem, or a mental health condition) that interferes with your performance as a student in this class, I encourage you to arrange support services and/or accommodations through Disabilities Services staff in the Seattle University Learning Center, Loyola 100, (206) 296‐5740. Disability‐based adjustments to course expectations can be arranged only through this process. Cell phones: Cell phones should be switched off or in silent mode throughout class. If you absolutely need to keep your phone on during class, you must notify the instructor prior to class. Examples would be: you have child‐care commitments or a close relative is in the hospital. Please be respectful of other students’ time and commitment to their studies by not breaching this policy so that we do not end up in the embarrassing position of having to ask you to leave class. If your cell phone does accidentally go off, turn it off immediately and do not answer the call. Text messaging is not allowed – it is detrimental to your learning and disruptive and disrespectful to the instructor and your classmates. Academic honesty: Cheating and plagiarism will not be tolerated in this course. The Seattle University academic honesty policy (see https://www.seattleu.edu/registrar/Policies.aspx) will be strictly enforced. If you are aware of a violation of policy, please inform an instructor. Transfer information: Please be advised that all colleges recommend you complete the entire sequence of general chemistry at one institution. If you plan to complete the sequence elsewhere, please let your professor and your academic advisor know. Not all general chemistry courses are equivalent. 4 CHEMISTRY 121‐05 Fall 2010 “Science is a way of thinking much more than it is a body of knowledge.” ‐Carl Sagan Learning and understanding a subject is a cumulative process, which requires active involvement of the student with the material and with others. Your learning resources for this course include the textbook, your class syllabus, your class notes, your completed assignment sets, classroom activities, OWL, your exams, other students in the class, your study group, students who have taken chemistry before, the Learning Center, and your professor. Lists of learning objectives for each chapter will be available on the course website. We recommend that you monitor your understanding of each chapter by referring to learning objectives often. Although many resources are available to you, your success will ultimately depend on effectively using these resources. For example, keeping your written materials for this course organized in a three‐ ring notebook makes it easier for you to access information when you need it. In addition, facilitated study groups will be offered through a cooperative effort of the Chemistry Department and the Learning Center. These study sessions function best when students attend regularly and come prepared. Finally, class time will be used to expand and clarify particularly important or difficult ideas and will not simply summarize every topic in the book. Therefore it is your responsibility to read designated chapters independently and ask questions if you do not understand a concept from the reading. Just like physical sports, academic learning is a form of exercise (for your brain), which requires repetition, practice, struggles, and reflection for real progress to occur. We have identified three areas, of mastery that are essential for success in understanding chemistry. They are language development, problem solving, and visualization. As you study, pay special attention to developing and implementing these skills. Fundamental understanding of chemistry and your ultimate progress in the course depend upon mastering the language of chemistry. The “key terms” list at the end of each chapter identify terms that you should know after studying a chapter. An essential starting point in learning any new language is memorization of words and definitions. However, simple memorization is not sufficient to promote understanding of concepts. The definitions given in the glossary of the text are often inadequate in conveying the scientific meaning of the term defined. Mature understanding of the language of chemistry requires that you find examples of defined vocabulary, discuss the meanings with your classmates, and apply definitions to explain important concepts. Problem solving is also an important feature of any chemistry course. Your text provides systematic strategies for approaching problems, which we will supplement during class. You should begin all problems by restating the given information and indicating what is to be found in the final answer to the problem. We will give you examples of how problems should be set up and expect that you follow that format on assignments and exams. Finally, the study of chemistry requires visualization. This includes picturing molecules in 2D and 3D, interpreting graphs, diagrams, and figures, making shorthand representations of physical systems, and drawing the process being described in a problem. Figures and illustrations in your text contain a great deal of information and should not be ignored. As you study, look at pictures and take time to create a mental or actual image of processes being described. We want you not only to succeed in this class, but also to become excited about chemistry! Start good study habits early, work with others, and don’t hesitate to contact us with questions and concerns as soon as they arise. 5