3.3 Phase Changes Phase changes: Energy Changes melting
advertisement

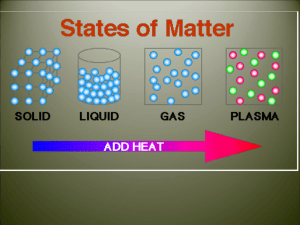
3.3 Phase Changes Phase changes: melting Energy Changes freezing vaporization condensation sublimation deposition The temperature of a substance does NOT change during a phase change. Energy is either released or absorbed during a phase change. Exothermic – Energy is released/given off to the surroundings Endothermic – Energy is taken/absorbed from the surroundings Melting and Freezing: The arrangement of molecules in water become more orderly as water freezes, and less orderly as ice melts. Melting – energy (heat) is absorbed to melt ice, so it is endothermic Freezing – energy (heat) is released, so it is exothermic Vaporization and Condensation: Vaporization – energy (heat) is absorbed to vaporize, so it is endothermic Condensation – exothermic Evaporation – takes place at the surface of a liquid and occurs at temperatures below the boiling point. Boiling – When the vapor pressure becomes equal to the atmospheric pressure Sublimation and Deposition: Sublimation – endothermic Deposition - exothermic