Malachite green - Akron Public Schools
advertisement

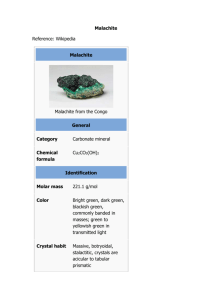
Malachite green • • • • • • Acryl brilliant green b ADC malachite green crystals Dimethyl chloride Aniline green Astra malachite green Astra malachite green b Formula C23H25ClN2 Structure Description Green crystals with metallic luster. Uses for directly dyeing silk, wool, jute and leather, dyeing cotton after mordanting. Biological stain, clinical reagent (inorganic phosphate assay). As spot test reagent for detecting sulfurous acid and cerium. Registry Numbers. CAS 569-64-2 EINECS 209-322-8 RTECS BQ1180000 EPA Pest. No. 39504 Merck Index 5739 569-64-2 is listed on the TSCA. Chemical and physical properties. Formula mass 364.92 Hazards and protection. Storage Store in a cool, dry place. Handling Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with skin and eyes. Keep container tightly closed. Avoid ingestion and inhalation. Protection Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators Follow the OSHA respirator regulations found in 29CFR 1910.134 or European Standard EN 149. Always use a NIOSH or European Standard EN 149 approved respirator when necessary. Small spills or leaks Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Stability Stable under normal temperatures and pressures. Incompatibilities No information available. Hazardous Decomposition Carbon monoxide, oxides of nitrogen, carbon dioxide. Fire related information. Fire fighting Wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Combustion generates toxic fumes. Extinguishing media: For small fires, use water spray, dry chemical, carbon dioxide or chemical foam. Health related information. Exposure effects Ingestion May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Inhalation The toxicological properties of this substance have not been fully investigated. Skin May cause skin irritation. Eyes May cause severe eye irritation. This product contains a cationic dye. Similar dyes have caused permanant injury to the cornea and conjunctiva in documented exposure cases with human or rabbit eyes. First aid Ingestion If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid. Inhalation Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear. Skin Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists. Eyes Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.