INSTRUCTIONS
®
Pierce FITC Labeling Kit
53004
1322.4
Number
Description
53004
Pierce FITC Labeling Kit, contains sufficient materials to perform five labeling reactions for
volumes of 50 μl to 1.0 ml using up to 10 mg/ml of protein for each reaction
Kit Contents:
No-Weigh™ Pre-Measured FITC (fluorescein isothiocyanate) Protein Labeling Reagent,
6 × 1 mg microtubes
Dimethylformamide (DMF), 1 ml
BupH™ Borate Buffer Packs, 5 packs, 50 mM borate, pH 8.5
BupH Phosphate Buffered Saline Packs, 5 packs, 0.1 M phosphate, 0.15 M sodium chloride; pH 7.2
D-Salt™ Dextran Desalting Columns with Column Extenders, 5 × 5 ml with 0.02% sodium azide
as a preservative
Slide-A-Lyzer® MINI Dialysis Units with Float, 5 units with 3,500 MWCO
Reaction Tubes, 5 each
Storage: Upon receipt store products at 4°C. Store fluorescent dye in foil pouch with desiccant to
protect from light and moisture. Store DMF in the resealable bag with desiccant to protect from
moisture. Kit is shipped at ambient temperature.
Introduction
The EZ-Label FITC Labeling Kit will label virtually any protein and is ideal for fluorescent labeling
of antibodies. The kit provides all of the reagents necessary to perform five labeling reactions using
up to 10 mg of protein per reaction.
FITC is among the simplest and most commonly used reagents for labeling proteins. The
isothiocyanate group on the fluorescein crosslinks with amino, sulfhydryl, imidazoyl, tyrosyl or
carbonyl groups on a protein; however, only derivatives of primary and secondary amines generally
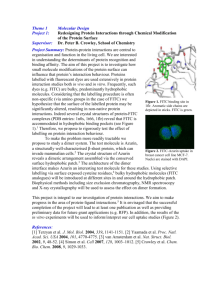
yield stable products. FITC has a molecular weight (MW) of 389 (Figure 1); excitation and emission
wavelengths at 494 nm and 520 nm, respectively; and a molar extinction coefficient of
72,000 M-1 cm-1 in an aqueous buffer, pH 8.
HO
O
O
OH
O
S
C
N
Figure 1. FITC chemical
structure.
Procedure
A. Protein Preparation
The optimal labeling buffer is BupH Borate Buffer at pH 8.5. Buffers that contain primary amines (e.g., Tris or glycine)
interfere with the intended FITC conjugation. Reconstitute one BupH Borate Buffer Pack with 500 ml of ultrapure water.
• If the protein is lyophilized and salt-free, prepare as follows:
Dissolve protein in the BupH Borate Buffer provided. For each labeling reaction use 50 μl to 1 ml of purified protein sample
at a concentration from 1-10 mg/ml. After reconstitution, proceed to Calculations section.
Pierce Biotechnology
PO Box 117
(815) 968-0747
3747 N. Meridian Road
Rockford, lL 61105 USA
(815) 968-7316 fax
www.thermo.com/pierce
• If the protein is already in a buffer (volume 100 μl or less), prepare as follows:
Protein must be exchanged into Borate Buffer by dialysis. The process for exchanging to Borate Buffer is similar to removing
excess fluorescent dye. For dialysis instructions, proceed to Removal of Excess Fluorescent Dye, For sample volumes of
100 μl or less (Section D). After the buffer has been exchanged, proceed to the Calculations section.
• If the protein is already in a buffer (volume greater than 100 μl), prepare as follows:
Protein must be exchanged into Borate Buffer by desalting. The process for exchanging to Borate Buffer is similar to
removing excess fluorescent dye. For desalting instructions, proceed to Removal of Excess Fluorescent Dye − For sample
volumes greater than 100 μl (Section D). After the buffer has been exchanged, proceed to the Calculations section.
B. Calculations
Perform the following calculations before beginning the Labeling Reaction.
The amount of FITC to use for each reaction depends on the amount of the protein to be labeled. By using the appropriate
molar ratio of FITC to protein, the extent of conjugation can be controlled. When conjugating proteins with FITC, a 24-fold
molar excess of the fluorescent dye is optimal; however, vary this ratio as needed to alter the degree of labeling. To
determine the amount of reconstituted dye to add to the protein sample, use the following equation:
1.
Calculate millimoles of FITC labeling reagent to add to the reaction:
ml protein ×
2.
mg protein mmol protein 24 mmol FITC
×
×
= mmol FITC
ml protein
mg protein
mmol protein
Calculate microliters of FITC solution to add to the reaction:
mmol FITC ×
389 mg
100 μl
×
= μl FITC
mmol FITC 1 mg
•
24 = Recommended molar ratio of FITC to protein
•
389 = Molecular weight of FITC
•
100 = Microliters of solvent in which the 1 mg of FITC is dissolved
EXAMPLE:
For 1 ml of a 1 mg/ml solution of IgG antibody (150,000 MW), 6.2 μl of the FITC is used.
Calculations:
1 ml IgG ×
24 mmol FITC
mmol IgG
1 mg IgG
×
×
= 0.00016 mmol FITC
mmol IgG
1 ml IgG 150,000 mg IgG
0.00016 mmol FITC ×
100 μl
389 mg
×
= 6.2 μl FITC
mmol FITC 1 mg
C. Labeling Reaction
Note: To protect reagents from moisture, allow the FITC and DMF to equilibrate to room temperature before opening.
1.
Transfer the protein solution to be labeled to a reaction tube.
2.
Reconstitute the FITC labeling reagent by puncturing the foil and adding 100 μl of DMF. Pipette up and down until the
FITC is completely dissolved.
3.
Transfer the appropriate amount of FITC (from Calculations section) to the protein-containing reaction tube.
4.
Mix well and incubate at room temperature for 1 hour.
Pierce Biotechnology
PO Box 117
(815) 968-0747
3747 N. Meridian Road
Rockford, lL 61105 USA
(815) 968-7316 fax
2
www.thermo.com/pierce
D. Removal of Excess Fluorescent Dye
For Sample volumes of 100 μl or less
Notes:
• Sample volumes up to 100 μl may be dialyzed in a single Slide-A-Lyzer MINI Dialysis Unit. For sample volumes of
100-200 μl, split the sample equally into separate dialysis units.
•
Add and remove sample using a standard laboratory pipette. To prevent contamination, do not touch the membrane.
•
Slide-A-Lyzer MINI Dialysis Units are disposable dialysis cups made of polypropylene and regenerated cellulose.
5.
Add sample to the Slide-A-Lyzer MINI Dialysis Unit. Cap the dialysis unit and place in a flotation device for dialysis.
6.
Float the dialysis unit in a container with at least 100 ml of PBS or other appropriate buffer and cover with foil to protect
FITC from light.
7.
Place beaker on a stir plate and stir. Use a low-speed setting on a stir plate so that the flotation device is not submerged.
8.
Dialyze for 1 hour at room temperature.
9.
Remove sample. For best sample recovery, tilt the unit and collect sample from the corner.
For sample volumes greater than 100 μl
10. Remove top cap from the desalting column and decant the storage solution.
11. Remove bottom cap from the column and equilibrate the column with 25 ml of PBS or other appropriate buffer.
12. Place column in a test tube and apply protein sample.
Note: For optimal separation, use sample volumes of 1 ml or less. To achieve optimal results, use more than one column
for sample volume of greater than 1 ml.
13. Allow sample to enter the desalting resin.
14. Place the column in a clean test tube and apply 0.5 ml of buffer. After the aliquot has flowed into the resin, transfer
column to a clean test tube. Repeat this step until protein has exited the column.
Note: To monitor the sample for protein content, measure the absorbance of each fraction at 280 nm. The first peak in
absorbance generally exits the column when 1 ml of buffer has been added after the sample is applied. This peak
contains protein and these fractions can be pooled. The non-reacted fluorescent dye exits the column in subsequent
fractions; discard these fractions after confirming that all fractions containing protein have been collected.
15. Regenerate the desalting columns by washing with 50 ml of PBS.
16. For storage, wash the column with 25 ml of PBS containing 0.02% sodium azide and cap the bottom then the top of the
column when approximately 2 ml of solution remains above the resin. Store columns at 4°C.
Additional Information
Please visit our website for additional information including the following:
•
Tech Tip: Calculate Dye:Protein (F/P) Molar Ratios
•
Tech Tip: Protein Stability and Storage
•
Tech Tip: Extinction Coefficients Guide
•
Tech Tip: Dialysis: An Overview
Pierce Biotechnology
PO Box 117
(815) 968-0747
3747 N. Meridian Road
Rockford, lL 61105 USA
(815) 968-7316 fax
3
www.thermo.com/pierce
Related Products
46110
FITC, 1 g
20673
Dimethylformamide (DMF), 50 ml
28384
BupH Borate Buffer Packs, 40 packs
28372
BupH Phosphate Buffered Saline Packs, 40 packs
43230
D-Salt Dextran Desalting Columns with Column Extenders
69550
Slide-A-Lyzer MINI Dialysis Units, 3,500 MWCO
53024
DyLight™ 488 Antibody Labeling Kit
53034
DyLight 549 Antibody Labeling Kit
53050
DyLight 649 Antibody Labeling Kit
53056
DyLight 680 Antibody Labeling Kit
53062
DyLight 800 Antibody Labeling Kit
General References
Goding, J. (1986). Monoclonal Antibodies: Principles and Practice, 2nd ed. Academic: London.
Larsson, L. (1988). Immunocytochemistry: Theory and Practice. CRC: Boca Raton, 77-83, 224-225.
Muramoto, K., et al. (1984). The application of fluorescence in isothiocyanate and high-performance liquid chromatography for the microsequencing of
proteins and peptides. Anal Biochem 141:446-50.
Pachmann, K., et al. (1991). Highly fluorochrome labeled gene probes for quantitative tracing of RNA in individual cells by in situ hybridization.
Bioconjugate Chem. 2:19-25.
Pallavicini, M., et al. (1989). Rapid screening and selection of monoclonal antibodies by bivariante flow cytometric analyses. J. Immunol Meth 117:99-106.
Karawajew, L., et al. (1990). Flow sorting of hybrid hybridomas using the DNA stain Hoechst 33342. J. Immuno. Meth. 129:277-82.
Spack, E., et al. (1986). Hydrophobic adsorption chromatography to reduce nonspecific staining by rhodaminelabeled antibodies. Anal Biochem 158:233-37.
Der-Balian, G., et al. (1988). Fluorescein labeling of Fab while preserving single thiol. Anal. Biochem. 173:59-63.
Lewinsohn, D., et al. (1988). A fluorometric approach to the quantitation of call number with application to a cell adhesion assay. J Immunol Meth 110:93100.
Cantinieaux, B., et al. (1989). Staphylococcus aureus phagocytosis: A new cytofluorometric method using FITC and paraformaldehyde. J Immunol Meth
121:203-8.
Porat, N., et al. (1988). Adenosine deaminase in cell transformation: biophysical manifestation of membrane dynamics. J Biol Chem 263(29):14608-11.
Champeil, P., et al. (1988). ATP regulation of sarcoplasmic reticulum Ca2+ ATPase. J Biol Chem 263(25):12288-94.
Horisberger, M. (1984). In Immunolabeling for Electron Microscopy. Polak, J. and Varndel, I. Eds. Elsevier: Amsterdam, 98.
Harlow, E. and Lane, D. (1988). Antibodies: A Laboratory Manual. Cold Spring Harbor Lab.: Cold Spring Harbor, New York, 409.
Slide-A-Lyzer MINI Dialysis Unit is protected by U.S. Patent # 6,039,871.
This product (“Product”) is warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale,
as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”) and to be free from defects in material and
workmanship. Unless otherwise expressly authorized in writing, Products are supplied for research use only. No claim of suitability for use in applications
regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the
Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This
warranty does not extend to anyone other than the original purchaser of the Product (“Buyer”).
No other warranties, express or implied, are granted, including without limitation, implied warranties of merchantability, fitness for any particular
purpose, or non infringement. Buyer’s exclusive remedy for non-conforming Products during the warranty period is limited to replacement of or
refund for the non-conforming Product(s).
There is no obligation to replace Products as the result of (i) accident, disaster or event of force majeure, (ii) misuse, fault or negligence of or by Buyer, (iii)
use of the Products in a manner for which they were not designed, or (iv) improper storage and handling of the Products.
Current versions of product instructions are available at www.thermo.com/pierce. For a faxed copy, call 800-874-3723 or contact your local distributor.
© 2008 Thermo Fisher Scientific Inc. All rights reserved. Unless otherwise indicated, all trademarks are property of Thermo Fisher Scientific Inc. and its
subsidiaries. Printed in the USA.
Pierce Biotechnology
PO Box 117
(815) 968-0747
3747 N. Meridian Road
Rockford, lL 61105 USA
(815) 968-7316 fax
4
www.thermo.com/pierce