Geology: The Geology of the Earth
advertisement
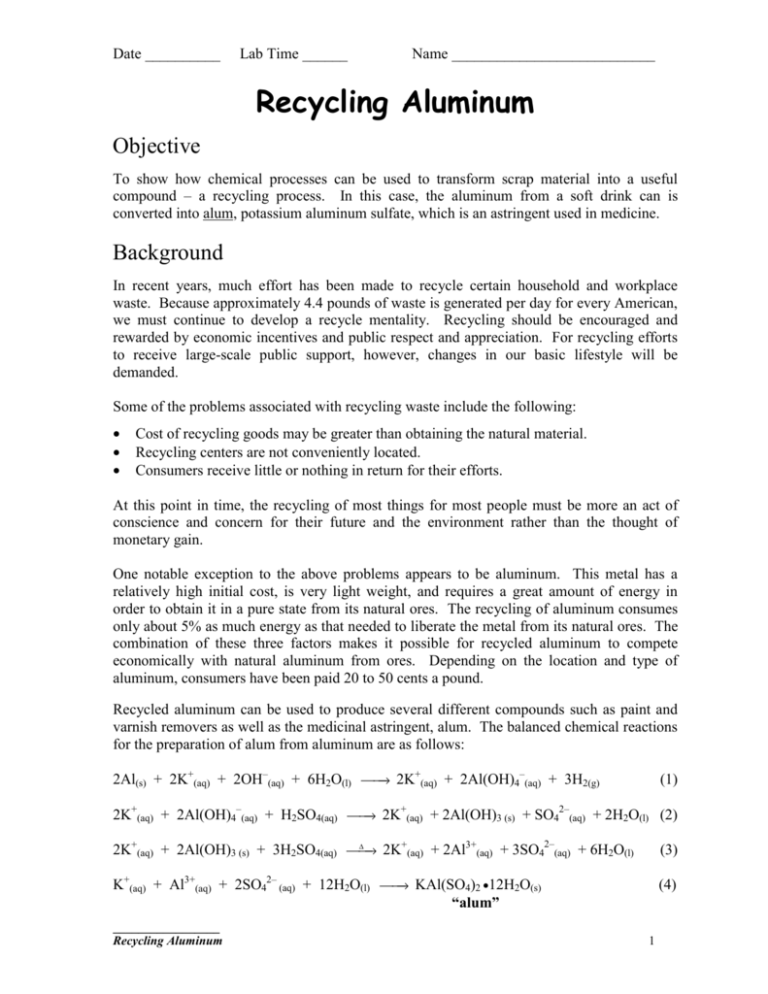
Date __________ Lab Time ______ Name ___________________________ Recycling Aluminum Objective To show how chemical processes can be used to transform scrap material into a useful compound – a recycling process. In this case, the aluminum from a soft drink can is converted into alum, potassium aluminum sulfate, which is an astringent used in medicine. Background In recent years, much effort has been made to recycle certain household and workplace waste. Because approximately 4.4 pounds of waste is generated per day for every American, we must continue to develop a recycle mentality. Recycling should be encouraged and rewarded by economic incentives and public respect and appreciation. For recycling efforts to receive large-scale public support, however, changes in our basic lifestyle will be demanded. Some of the problems associated with recycling waste include the following: • • • Cost of recycling goods may be greater than obtaining the natural material. Recycling centers are not conveniently located. Consumers receive little or nothing in return for their efforts. At this point in time, the recycling of most things for most people must be more an act of conscience and concern for their future and the environment rather than the thought of monetary gain. One notable exception to the above problems appears to be aluminum. This metal has a relatively high initial cost, is very light weight, and requires a great amount of energy in order to obtain it in a pure state from its natural ores. The recycling of aluminum consumes only about 5% as much energy as that needed to liberate the metal from its natural ores. The combination of these three factors makes it possible for recycled aluminum to compete economically with natural aluminum from ores. Depending on the location and type of aluminum, consumers have been paid 20 to 50 cents a pound. Recycled aluminum can be used to produce several different compounds such as paint and varnish removers as well as the medicinal astringent, alum. The balanced chemical reactions for the preparation of alum from aluminum are as follows: – + 2Al(s) + 2K+(aq) + 2OH–(aq) + 6H2O(l) → 2K (aq) + 2Al(OH)4 (aq) + 3H2(g) (1) 2– + 2K+(aq) + 2Al(OH)4–(aq) + H2SO4(aq) → 2K (aq) + 2Al(OH)3 (s) + SO4 (aq) + 2H2O(l) (2) 2– + 3+ ∆ 2K+(aq) + 2Al(OH)3 (s) + 3H2SO4(aq) → 2K (aq) + 2Al (aq) + 3SO4 (aq) + 6H2O(l) (3) → KAl(SO4)2 •12H2O(s) K+(aq) + Al3+(aq) + 2SO42– (aq) + 12H2O(l) “alum” (4) _________________ Recycling Aluminum 1 Date __________ Lab Time ______ Name ___________________________ From the balanced equations it can be seen that 2 moles of aluminum (in the first equation) yield 2 moles of alum (in the third equation). Thus, one mole of aluminum will produce one mole of alum. Using this information, the amount of alum that should be able to be produced from a given starting amount of aluminum can be determined. This is called the theoretical yield and is determined for alum by performing the following calculation for X grams of aluminum: 1 mole Al 1 mole alum 424 g alum theoretical yield alum ( g ) = X g Al 27 g Al 1 mole Al 1 mole alum It is often the case, however, that the actual amount of alum produced in an experiment does not match this theoretical value. Knowing the theoretical yield and the actual yield, it is possible to determine the percent yield using the following relationship: % yield = actual yield x 100% theoretical yield Materials needed aluminum can scissors 100 mL graduated cylinders 250 mL beaker (2) filter paper filter funnel glass wool hot plate beaker tongs Büchner funnel 2 M potassium hydroxide (KOH) 9 M sulfuric acid (H2SO4) distilled water bottles ice 50/50 ethanol-water mixture suction flask/vacuum hose rubber policeman Procedure 1. Obtain approximately 1 g of aluminum scrap from an aluminum soft drink can and use a balance to measure and record the exact mass to the nearest 0.001 g. Cut the aluminum scrap into very small pieces and put them in a clean 250 mL beaker. 2. Set up a hot plate in the fume hood. Using a 100 mL graduated cylinder, add 50 mL of 2 M potassium hydroxide, KOH, to the aluminum pieces in the 250 mL beaker. CAUTION: DO NOT SPLATTER THE SOLUTION. Place the 250 mL beaker on the hot plate. Heat the beaker and stir gently. Hydrogen gas will be evolved at this point so be certain the fume hood is turned on with the glass shield pulled at least halfway down. _________________ Recycling Aluminum 2 Date __________ Lab Time ______ Name ___________________________ 3. After approximately 10-20 minutes the aluminum should be mostly dissolved, except for small amounts of impurities floating at the top. Continue heating until the fizzing around each piece stops. [*** If the liquid level drops to ¼ the original volume, add distilled water to bring the level back to ½ the original volume. This may be repeated several times before all of the aluminum dissolves.] 4. Using beaker tongs, remove the beaker from the hot plate and carefully filter the contents through glass wool in a filter funnel. Recover the filtered solution in another clean 250 mL beaker and allow it to cool, stirring occasionally. 5. Using a graduated cylinder, slowly and carefully add 20 mL of 9 M sulfuric acid, H2SO4, to the solution in the beaker. Lumps of aluminum hydroxide, Al(OH)3, will form and will be dissolved as the H2SO4 is added (equations 2 and 3 in Background). Using the hot plate, gently heat the new mixture for about 10-15 minutes to completely dissolve all the Al(OH)3. 6. After the solution becomes clear, remove it from the heat and cool it in an ice bath for 15 minutes. Stir slowly while scratching the bottom of the beaker lightly to aid in crystal formation. After cooling and stirring, alum crystals should be present in the reaction beaker. *** While the mixture is cooling, set up the Büchner funnel and suction flask. Place a piece of filter paper in the funnel top. Weigh the funnel top and a slightly moistened piece of filter paper together and record the mass on the report sheet. 7. Turn on the water to start the suction process. Place the filter paper in the Büchner funnel and add some water to wet the filter paper. Carefully transfer the alum crystals to the filter paper in the Büchner funnel, scraping the beaker with the rubber policeman to remove all the crystals. Wash the beaker with a squirt bottle (a little bit at a time) of a 50/50 ethanol-water mixture (in which alum is not very soluble) until all of the remaining crystals have been emptied into the funnel top. 8. Allow the crystals to be sucked dry for several minutes in the funnel (until dripping stops). When the crystals are dry, remove the hose before turning off the water. Remove the funnel top and measure the mass of the funnel top with the filter paper and alum crystals. Record results on the report sheet. 9. Determine the mass of the alum crystals. Disposal Procedure ** Place the crystals in the collection bottle provided ** Place the used glass wool and filter paper in the trash. ** Clean all equipment at the sink. Dry all used equipment and return it to your station. _________________ Recycling Aluminum 3 Date __________ Lab Time ______ Name ___________________________ Report Sheet – Data Analysis Mass of aluminum _______________ g Mass of filter paper + Funnel Top _______________ g Mass of alum crystals + filter paper + Funnel Top _______________ g Mass of alum crystals (actual yield) _______________ g Calculate THEORETICAL YIELD AND % YIELD in the space below. Theoretical Yield _______________ g % Yield _______________ % Post-lab questions: 1. A student weighs his alum and finds that the actual yield is far less than the theoretical yield. Suggest two reasons (sources of error) why this might happen. 2. A student finds that her actual yield is greater than her theoretical yield. Suggest two reasons (sources of error) why this might happen. _________________ Recycling Aluminum 4 Date __________ Lab Time ______ Name ___________________________ Summary/Conclusions: _________________ Recycling Aluminum 5







