Threatened tadpoles of Bokermannohyla alvarengai
advertisement

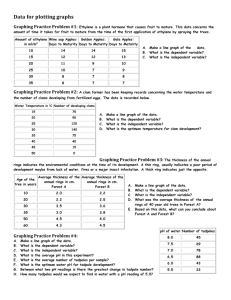
Biological Journal of the Linnean Society, 2010, 101, 437–446. With 1 figure Threatened tadpoles of Bokermannohyla alvarengai (Anura: Hylidae) choose backgrounds that enhance crypsis potential PAULA C. ETEROVICK1*, FRANCISCO F. R. OLIVEIRA1 and GLENN J. TATTERSALL2 1 Programa de Pós-Graduação em Zoologia de Vertebrados, PUC Minas, Belo Horizonte, 30535-610, Minas Gerais, Brazil 2 Department of Biological Sciences, Brock University, St Catharines, Ontario, Canada Received 13 April 2010; revised 15 April 2010; accepted for publication 21 April 2010 bij_1501 437..446 Crypsis results from a complex interaction among prey coloration, background matching, behaviour and predator visual perception. Tadpoles are known to have varied adaptations to escape predation, but the use of crypsis is little explored, although it is likely for certain species. We investigated potential escape mechanisms related to active escape (fleeing) and crypsis improvement in Bokermannohyla alvarengai tadpoles, proposing a new method to measure cryptic potential. We studied the range of distances covered by threatened and fleeing tadpoles and the proportion of tadpoles that seek shelter or remain exposed after fleeing. We hypothesized that tadpoles that remain exposed may use alternative strategies to avoid detection, such as reaching deeper microhabitats or positioning themselves on substrates that confer greater crypsis than the ones they were on before disturbance. A significantly greater proportion of tadpoles remained exposed after disturbance and positioned themselves on backgrounds that offered greater cryptic potential, but did not differ in depth. Tadpoles may respond to a trade-off between sheltering and being cryptic. On the one hand, they may remain close to retreat sites or they may escape to microhabitats that provide appropriate background matching as a means to achieve crypsis. On the other hand, the absence of matching backgrounds in the tadpoles’ vicinities may induce them to seek shelter. © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446. ADDITIONAL KEYWORDS: background choice – background matching – escape strategy. INTRODUCTION Defensive coloration is widespread among animals and may work to reduce predator detection risk (camouflage), warn predators of potential harm through unpalatable or toxic chemicals in the prey’s body (aposematism) or mislead predators into mistaking the prey for something else (mimicry) (Endler, 1978; Stevens, 2007; Stevens & Merilaita, 2009). Among these, camouflage constitutes a complex subject and may include crypsis (when prey detection is prevented, for instance, through background matching, disruptive coloration or distractive markings), masquerade responses (where the prey resembles some component of the background, such as a leaf), motion *Corresponding author. E-mail: eterovick@yahoo.com dazzle or motion camouflage (which impair estimates of speed and trajectory or movement detection by the predator) (Endler, 2006; Stevens & Merilaita, 2009). A combination of background matching and disruptive patterns is expected to provide the best means of avoiding detection, especially in prey that use many different backgrounds (Endler, 2006). However, according to Merilaita & Lind (2005), cryptic potential may vary, depending on what part of the background the prey matches, so that resembling a random part of the background is not necessarily sufficient to achieve crypsis. Clearly, predator visual perception is crucial for defensive colour mechanisms to be effective (Stevens, 2007). Visual orientation is usually important in vertebrate predators, so they are prone to being deceived by and/or responding to defensive colorations. For instance, an increase in edge profiles, resulting from © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 437 438 P. C. ETEROVICK ET AL. disruptive coloration, may prevent frog detection by the garter snake Thamnophis sirtalis (Osorio & Srinivasan, 1991). In tadpoles, behavioural defences against predators include spatial avoidance, hiding and decreased activity level (Teplitsky & Laurila, 2007). Morphological changes may be induced by predators and vary depending on the type of predator, being more efficient against the inducing type (Relyea, 2003; Benard, 2006). Alternatively, greater investment in growth (by increased feeding activity) may lead to a size refuge or early metamorphosis, decreasing predation risk as well (Van Buskirk, 2000). Defence mechanisms in tadpoles constitute an interesting study subject. However, crypsis in tadpoles is little explored. Predator-induced colour changes in Hyla chrysoscelis tadpoles have been interpreted as a potential disruptive coloration (McCollum & Leimberger, 1997), but they probably have the same effect as increased tail height (also an inducible defence in tadpoles) in misdirecting predator strikes to the tail and away from the vital body area (Van Buskirk et al., 2003; Richardson, 2006). Tadpoles in open Cerrado and montane meadow streams are preyed upon by aquatic insects, such as Heteroptera (Belostomatidae) and Anisoptera (Eterovick & Barata, 2006). Tadpoles of some species seek shelter to decrease predation risk (Kopp, Wachlevski & Eterovick, 2006), while others, however, remain exposed most of the time, resting on the bottom substrate (Eterovick & Sazima, 2004). Although aquatic insects are known to use movement as a cue to locate prey (e.g. Victor & Ugwoke, 1987), which induces reduced movement responses in tadpoles (Skelly & Werner, 1990), visual stimuli may also be important (e.g. Victor & Ugwoke, 1987). In fact, tadpoles of Hyla chrysoscelis exhibit changes in colour, shape and growth rate in response to the presence of dragonfly nymphs (McCollum & Leimberger, 1997); these induced morphological changes are efficient at reducing predation (McCollum & Van Buskirk, 1996). Besides aquatic predators, it is known that tadpoles may also be preyed upon by birds and snakes in Cerrado/montane meadow habitats (Kopp & Eterovick, 2006). Such vertebrate predators may use visual cues to detect tadpoles, so defensive colorations might evolve as a mechanism to avoid detection in palatable species. Both terrestrial reptiles and birds retain the five ancestral sets of visual pigments, including four cone classes with different spectral sensitivities, which allow for the efficient coding of spectral information, meaning that colour vision is likely to be important for object detection and classification (Osorio & Vorobyev, 2005). Bokermannohyla alvarengai (Bokermann, 1956) is a large tree frog (adult males reach 110 mm snout- vent length) restricted to the Espinhaço mountain range, south-eastern Brazil (Bokermann, 1956; Eterovick & Sazima, 2004). The adults are nocturnal and they may rest on rocks exposed to direct sunlight during the day (Tattersall, Eterovick & Andrade, 2006). Their coloration resembles lichens, conferring effective crypsis through background matching (Sazima & Bokermann, 1977; Eterovick & Sazima, 2004; Tattersall et al., 2006) that may help them avoid detection by visually oriented predators. Bokermannohyla alvarengai breeds during the rainy season in temporary streams where it lays aquatic egg clutches of c. 1500 eggs. The tadpoles are diurnal and dwell in backwaters, usually on submerged rocks. Their development takes approximately 4 months. They are relatively small, attaining a maximum size of 53 mm total length (Sazima & Bokermann, 1977), have no bright colours likely to indicate unpalatability and remain in the streams for several months, exposed to diurnal, visually oriented predators. Combined, these traits ensure that they constitute a good model to study strategies used by tadpoles that remain exposed in shallow montane meadow temporary streams, where detection by terrestrial vertebrates, especially birds and snakes, is likely. In order to understand how B. alvarengai tadpoles manage to survive a potentially high predation risk from visually oriented predators, we investigated potential escape mechanisms related to active escape (fleeing) and camouflage. We first studied the range of distances covered by fleeing tadpoles and the proportion of tadpoles that sought shelter or remained exposed after fleeing. We hypothesized that tadpoles that remained exposed after fleeing would use alternative strategies to avoid detection, such as (1) reaching deeper microhabitats or (2) positioning themselves on substrates that confer greater background matching than the ones they were on before disturbance. We assumed that deeper microhabitats might make tadpoles harder to detect for terrestrial predators and also give them a little extra time to escape once detected. MATERIAL AND METHODS STUDY SITE The Rio Preto State Park (43°30′676″W; 18°00′799″S) is located in the state of Minas Gerais, on the southern–central portion of the Espinhaço mountain range, in south-eastern Brazil. It encompasses vegetation types belonging to the Cerrado biome, including montane meadows. The climate is tropical wet (sensu Ab’Saber, 1977), with two well-defined seasons: a wet, warm season from October to March and a dry, cold © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 CRYPSIS OF THREATENED TADPOLES season from April to September. Annual rainfall in the region averaged 1351.22 mm from 1995 to 2003. Air temperatures and humidity measurements gathered during a 1-year study at two rivers in the park were within the ranges of 8.5–38.0 °C and 20.0–89.0%, respectively (Oliveira & Eterovick, 2009). The present study was carried out in seasonal streams formed during the wet season, which run amidst rocky outcrops and bushy fields, at elevations of between 1300 and 1400 m. These temporary streams shelter no fish, but there are birds (e.g. Pitangus sulphuratus, Tyrannidae) and snakes (e.g. Xenodon merremii, Liophis poecilogyrus, L. miliaris, Helicops modestus, Dipsadidae, sensu Zaher et al., 2009) in the area that can potentially prey on tadpoles (A. M. Souza, pers. comm.). There are also reports in the literature of some of these species feeding on tadpoles in other areas (e.g. Pitangus sulphuratus; Kopp & Eterovick, 2006; Helicops modestus; Sawaya, Marques & Martins, 2008) and one of us (F.F.R.O.) saw an individual of Liophis sp. inside one of the sampled streams. TADPOLE SAMPLING We sampled tadpoles of Bokermannohyla alvarengai in seven montane meadow streams with heterogeneous bottoms containing sand, rocks, some leaf litter, submerged grasses and pebbles, all forming a mosaic. The extent of sampling and sampled area varied among streams, according to B. alvarengai tadpole presence. The water was very clear and the sampled sections exhibited low depth values 439 (1–20 cm), allowing us to detect all tadpoles present through careful examination of the whole bottom. Deeper sections occur sporadically among rock crevices and we may have missed some tadpoles that were originally hidden. However, we searched such microhabitats with nets and found no tadpoles, indicating that they were usually exposed before we disturbed them (as described below). We collected data on tadpole escape behaviour in one stream on December 2006 and in another six streams in November and December 2008 (Table 1). Based on 32 measured specimens, which we believe are representative of our whole sample, tadpole total size varied from 43 to 52 mm and tadpole developmental stage varied from 27 to 41 (sensu Gosner, 1960). Tadpole behaviour may change with developmental stage, although it was not our aim to test such variation in the present study. For this purpose, it would be appropriate to have a representative sample of tadpoles in stages distributed through the whole developmental period, which was not the case, as we found tadpoles that could not have varied by more than 20% of total body length in the field. The maximum size of tadpoles of B. alvarengai is attained in stage 40 (53 mm total length, Sazima & Bokermann, 1977). We carefully photographed each tadpole located before it made any attempt to escape and then one of us moved a finger from above (P.C.E. in December 2006 and F.F.R.O. in November and December 2008) slowly and continuously towards the tadpole, inside the water, until a escape response was observed (it always happened before the finger touched the tadpole). This Table 1. Mean depths of microhabitats occupied by Bokermannohyla alvarengai tadpoles before and after disturbance, depth change (depth after–depth before) and distance covered when disturbed, by sampling dates and streams Streams S1 S2 S3 S4 S5 S6 S7 Sampling periods December 2006 November 2008 December 2008 Totals (overall means) Sample size*: nexp (nphoto), nshelter Coordinates 18°11′19″S, 18°11′36″S, 18°12′42″S, 18°11′35″S, 18°12′39″S, 18°11′17″S, 18°08′09″S, 43°20′14″W 43°20′08″W 43°20′07″W 43°20′08″W 43°20′07″W 43°20′13″W 43°20′43″W Streams sampled S7 S1–S6 S1–S5 Depth before (cm) Depth after (cm) Depth change (cm) Distance covered (cm) (33), 12 (8), 3 (6), 1 (1), 1 (9), 1 (5), 1 (7), 10 9.59 ± 4.28 8.00 ± 4.00 9.29 ± 3.30 11.25 ± 4.35 8.23 ± 5.22 4.00 ± 1.41 9.46 ± 3.59 10.00 ± 4.82 8.50 ± 4.43 8.43 ± 4.08 7.50 ± 3.32 8.53 ± 5.61 4.50 ± 1.64 9.60 ± 3.68 0.42 ± 5.19 0.50 ± 2.31 -0.86 ± 4.45 -3.75 ± 7.32 0.29 ± 6.26 0.50 ± 2.88 0.14 ± 3.25 9.51 ± 3.91 10.07 ± 6.01 10.86 ± 4.06 12.00 ± 5.48 10.06 ± 3.86 11.00 ± 2.00 25.37 ± 17.84 21 (7), 10 30 (7), 7 59 (55), 12 9.46 ± 3.59 4.37 ± 1.96 10.98 ± 3.55 9.60 ± 3.68 4.77 ± 2.32 10.97 ± 4.34 0.14 ± 3.25 0.40 ± 2.21 -0.02 ± 5.91 25.37 ± 17.84 9.20 ± 3.04 10.44 ± 4.67 110 (69), 29 8.86 ± 4.29 8.99 ± 4.62 0.13 ± 4.73 12.36 ± 9.58 41 14 7 4 17 6 21 *All values refer to tadpoles that remained exposed after attempting to escape (nexp) and are given as mean ± SD. The sample sizes presented include tadpoles that did not seek shelter after disturbance and remained exposed (nexp); among these, the number of tadpoles that could be successfully photographed both before and after disturbance (nphoto) and the number of tadpoles that sheltered themselves after disturbance (nshelter). © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 440 P. C. ETEROVICK ET AL. stimulus was used to elicit a escape response in a controlled way that would allow us to make minimal disturbance in the water surface and thus observe where all tadpoles went after disturbed. Different predators may have different approaches and elicit different responses and whether escape speed or covered distance vary depending on type of predator is an interesting subject that deserves further study. But our aim here was to study tadpole behaviour in response to a standardized frightening stimulus. A similar approach has already been used to study choice of cryptic backgrounds by grasshoppers (Eterovick, Figueira & Vasconcellos-Neto, 1997). We recorded whether the tadpole was exposed or sheltered after moving away from the disturbance and, whenever it was exposed, we took another photograph. We then measured with a metric tape and recorded the distance covered by the tadpole from its initial to its final position, the depth of both positions and the distance from the tadpole’s initial position to the closest structure that could potentially provide some shelter (under which the tadpole could hide). We took these measurements in situ, after we were finished photographing the tadpoles. These structures included rock crevices, submerged herbs or leaf litter (we considered whichever was the closest to the tadpole’s original position). Movements were usually straight, but in a single instance a tadpole made a curved trajectory, which we considered in determining its escape distance. We moved upstream while making observations to avoid sampling the same tadpole more than once. In all sampling periods we conducted tadpole observations while natural light allowed good visualization of tadpoles (between 11:00 and 17:00 h). We discarded pictures that were blurry or had light reflections that interfered with tadpole or background colour visualization. We took pictures of tadpoles with an automatic Sony Cyber Shot DSC-P100 camera with Carl Zeiss lenses, 3 ¥ optical zoom, 5.1 megapixels in December 2006 and with a Canon Powershot A630 (in automatic mode, with 2.8 aperture and 1/13–1/40 speed) in November and December 2008. We positioned the cameras almost perpendicular to the water surface, but somewhat at an angle to minimize reflections while still providing a dorsal view of the tadpole. We tested for differences between sampling periods, which would also account for differences between cameras when conducting colour measurements and analyses (see ‘Colour measurement and analyses’). According to Stevens et al. (2007), calibration for calculating reflection from digital photographs needs to be performed for each session/light, because the light set-up may change the ratio between different wavelengths (long, medium or short waves). We did not perform such calibration during our fieldwork because we were comparing pic- tures of the same tadpole which were taken less than 30 s apart, so there was likely not enough time for light conditions to change. Additionally, even if we considered that light conditions could still change, any distortion caused in a particular wavelength would be corrected for when we calculated the differences between the tadpole and the background it was on, because any detection-based distortion in the wavelengths would be the same for the tadpole and its background. Furthermore, the tadpole was the same individual in both pictures whose difference between tadpole/background would be compared (see ‘Colour measurement and analyses’). This way, the tadpole itself worked as a calibration for background colour when differences between tadpole and background were calculated. Furthermore, any inter-individual variation in the colour estimation of the images would have decreased the chances of detecting significant differences by increasing the error term in our statistical analysis. TADPOLE MICROHABITAT DEPTH AND ESCAPE DISTANCE We tested whether proportions of tadpoles escaping to shelters and remaining exposed varied among different streams using a c2-test (7 ¥ 2 contingency table, Ayres et al., 2005) to account for a possible effect of local stream features or population on tadpole response to disturbance. We then compared the mean number of tadpoles sheltered and exposed using a Mann–Whitney U-test to assess whether tadpoles exhibited a preferential response to disturbance. We compared the distance from a tadpole’s initial position to the closest potential shelter (rock crevice, leaf litter or aquatic vegetation) between tadpoles that used and that did not use these shelters with a Mann–Whitney U-test. We compared data on depth (both before and after disturbance), depth variation between microhabitats occupied before and after disturbance and distance covered by tadpoles during escape between sampling dates and among streams using Kruskal–Wallis tests. We wanted to know whether sampling date or stream influenced the estimated parameters or not; if not, we could consider dates and streams as replicates and pool all data for subsequent analyses. We then compared depths of microhabitats used before and after disturbance using a Wilcoxon test to test the hypothesis that disturbed tadpoles would escape towards deeper microhabitats. We used Shapiro– Wilk tests to test for data normality and chose statistical tests accordingly. We conducted all statistical tests using the software Systat (Systat Software, 2007). © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 CRYPSIS OF THREATENED TADPOLES COLOUR MEASUREMENT AND ANALYSES To test whether tadpoles that remained exposed were maximizing their cryptic potential through background matching, we compared their colour with background colour both in their initial position and in their position after escaping. Background vs. prey texture (colour patch size) variation is also important for camouflage (e.g. Shohet et al., 2007). When patches of the background that exhibit noticeable colour variation are smaller than the prey, it requires a sufficiently accurate match to achieve good camouflage (Merilaita, 2003). But, in the present study, we did not make any measurement of texture. Most substrates (rocks, sand) 441 were relatively uniform and similar in colour to the tadpoles’ bodies. A few tadpoles positioned themselves on mosaic substrates such as pebbles, but as the texture of such substrates was not smaller than the tadpole’s bodies, we considered that matching parts of the substrate would still provide camouflage (for examples of substrates, see Fig. 1). The backgrounds used by these tadpoles are mottled, with grain size similar to the colour grain size in tadpoles’ bodies. The exceptions are rare pebbles/rocks, which can be seen in Figure 1, that shows examples of the different types of substrates observed. Transitions between colours in the background or in the tadpoles were rarely abrupt and, even when light and dark Figure 1. Pictures of Bokermannohyla alvarengai tadpoles showing an example of tadpole position (A) before and (B) after disturbance; layers created in Adobe Photoshop for colour analyses of (C) tadpole and (E) background; and examples of tadpoles on (D) sandy and (F) rocky substrates. Notice the similarity between tadpole and background texture. The two light dots on tadpole dorsum (arrows) may function as distractive markings against light backgrounds. Scale bar, 10 mm (A). © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 442 P. C. ETEROVICK ET AL. tones were present, there were always intermediate shades in the background mosaic, so we believe the method we used was appropriate to show tadpole cryptic potential on their background. Although we are not specifically looking at disruptive patterns, a tadpole sitting amongst alternating contrasting backgrounds would be difficult for any animal to see in the type of habitat where they occur. Under such conditions, in order for a tadpole to be visible, the lightness or amount of different wavelengths should be, in general, lighter or darker in its background and our method would detect this situation. Colour analyses can be performed in several colour spaces, which consist of Cartesian spaces where the visual sensation of a colour can be uniquely defined by a set of numbers representing chromatic features (Drimbarean & Whelan, 2001). The ‘Lab’ colour space was originally proposed by Hunter (1948) and modified by the International Commission on Illumination (CIE, 1986) to what is currently called ‘CIE L*a*b*’. The ‘L*’ component refers to a scale of lightness (low values are dark, whereas high values are light), whereas the ‘a*’ and ‘b*’ components represent colour changes through the red–green and the yellow–blue spectra, respectively. For an appropriate colorimetric test, the lightness component must be analysed separately from the a*b* component, as the lightness is critical for the spatial formation of the image (McCormick-Goodhart & Wilhelm, 2003). In computer image manipulation, a* and b* can be adjusted in levels corresponding to 6-unit increments resulting in changes noticeable to the human eye, without any interference in L*. Their simultaneous adjustment, that is, a combined analysis of both a* and b*, can be defined as Da*b* = √(Da*2 + Db*2) (McCormick-Goodhart & Wilhelm, 2003). We used Adobe Photoshop CS2, version 9.0.2 (Adobe Photoshop 1999–2005, Adobe Systems Incorporated) to quantify L*, a* and b* components in the pictures of Bokermannohyla tadpoles before and after disturbance. We assessed the components in the tadpoles by selecting them as a separate layer (Fig. 1). In order to assess background components, we arbitrarily marked five elliptical shapes throughout the background adjacent to the tadpole, merged them in a separate layer and proceeded to a component measurement of the whole set of five elliptical shapes, to obtain a general background measurement (Fig. 1). We marked elliptical shapes close to tadpole body shape and size and we tried to encompass all substrates surrounding the tadpoles in the available proportions. We avoided parts of the pictures that eventually had light reflections on water surface or the tadpole’s shadow. We used the means of L*, a* and b* components to calculate |DL*| and Da*b* for tadpole vs. background colour before and after disturbance, as follows: ΔL* before = L* bb − L*tb ; ΔL* after = L* ba − L*ta ; Δa*b* before = [(a*bb − a*tb)2 + (b* bb − b*tb)2 ]; Δa*b*after = [(a* ba − a*ta)2 + (b* ba − b*ta)2 ]; where bb indicates background before tadpole disturbance, tb is tadpole before disturbance; ba is background after tadpole disturbance and ta is tadpole after disturbance. We performed paired t-tests to check whether tadpoles moved towards backgrounds with lightness (L*) and colours (a*b*) more similar to their own. We compared |DL*| and Da*b* among streams and among sampling periods using Kruskal–Wallis tests. Differences in L*, a* and b* among pictures are likely to be caused by light differences throughout the day. However, by always using the difference in brightness and colour between tadpole and background, which were in the same picture, we believe these variations were controlled. Besides, we considered that this variation is part of the natural context of camouflage, as predators may attack at different times and under different light conditions. RESULTS When disturbed, tadpoles of B. alvarengai always reacted by fleeing away from the stimulus, but they usually covered a short distance (12.36 ± 9.58 cm; Table 1). After this initial swimming burst, tadpoles either retreated to shelters or remained exposed and motionless. The proportion of tadpoles that sheltered themselves and that remained exposed after disturbance did not change significantly among streams (c2-test = 5.73, d.f. = 6, P = 0.455), so we could pool samples from all streams to compare numbers of tadpoles sheltering and not sheltering. A significantly greater number of tadpoles remained exposed (110 out of 139) after attempting to escape from a potential threat (Mann–Whitney U-test statistic = 6.00, P = 0.017). The tadpoles that moved to shelters when disturbed (29 out of 139) were significantly closer to potential shelters before disturbance than the ones that remained exposed after disturbance (Mann– Whitney U-test statistic = 874.50, P = 0.016). The shelters most frequently used were crevices among rocks (23 tadpoles), followed by leaf litter (three tadpoles) and aquatic vegetation (three tadpoles), which was in accordance with a higher availability of rock crevices as potential shelters (among 108 records of the type of © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 CRYPSIS OF THREATENED TADPOLES shelter closest to the tadpole, 97 were rock crevices, eight were leaf litter and four were aquatic vegetation). The proportional use of the three types of shelter was not significantly different from expected based on shelter availability, indicating no preference by the tadpoles (c2-test = 2.51, d.f. = 2, P = 0.284). Depths of microhabitats occupied by tadpoles before and after disturbance varied among sampling dates (depth before: Kruskal–Wallis test statistic = 50.68, P < 0.001; depth after: Kruskal–Wallis test statistic = 43.46, P < 0.001) but not among streams (depth before: Kruskal–Wallis test statistic = 12.15, P = 0.059; depth after: Kruskal–Wallis test statistic = 10.43, P = 0.108). However, the depth difference between microhabitats occupied before and after disturbance was not significant among sampling dates (Kruskal–Wallis test statistic = 0.406, P = 0.816) or streams (Kruskal–Wallis test statistic = 1.115, P = 0.981), so we pooled data from all dates and streams to test whether tadpoles move to deeper microhabitats when disturbed. Distances covered by tadpoles when disturbed varied among sampling dates (Kruskal–Wallis test statistic = 17.36, P < 0.001) and among streams (Kruskal–Wallis test statistic = 18.23, P < 0.001). The distances covered were longer in the stream sampled in December 2006 (S7, Table 1). We conducted the same analysis, excluding data from this stream (all from December 2006), and the distances no longer varied among sampling periods (Mann– Whitney U-test statistic = 744.50, P = 0.221) or streams (Kruskal–Wallis test statistic = 2.69, P = 0.747). When disturbed, tadpoles did not move to deeper microhabitats (Z = 0.297, P = 0.766). However, they moved to backgrounds significantly more similar to them in lightness (mean |DL*|before = 32.42 and mean |DL*|after = 25.37; t = 3.63, d.f. = 68, P < 0.001) and in colour (mean Da*b* before = 8.50 and mean Da*b*after = 7.39; t = 1.70, d.f. = 68, P = 0.046). For colour analyses, we also pooled data from all sampling periods as there was no significant difference among them (|DL*|before: Kruskal–Wallis test statistic = 3.04, P = 0.218; |DL*|after: Kruskal–Wallis test statistic = 1.32, P = 0.518; Da*b*before: Kruskal–Wallis test statistic = 5.41, P = 0.067; Da*b*after: Kruskal– Wallis test statistic = 2.96, P = 0.228). There was no significant difference among streams in |DL*|before (Kruskal–Wallis test statistic = 6.10, P = 0.412), |DL*|after (Kruskal–Wallis test statistic = 3.79, P = 0.705) or Da*b*after (Kruskal–Wallis test statistic = 9.90, P = 0.129). Da*b*before was significantly different among streams (Kruskal–Wallis test statistic = 19.73, P = 0.003), but we still pooled the data from all streams to make subsequent tests more robust. We were mostly interested in the tadpole’s escape behaviours, which were expressed after disturbance, when 443 there were no significant differences in the measured parameters (|DL*|after and Da*b*after) among streams. DISCUSSION Tadpoles of B alvarengai attempted to escape the threatening stimulus imposed on them by fleeing and either sheltering themselves or remaining exposed and motionless. The availability of suitable shelters close to the tadpole may be important in determining the outcome of the escape strategy, as tadpoles that chose to shelter were initially closer to potential shelters than tadpoles that remained exposed. In these circumstances, tadpoles seem to use any type of shelter, showing no preferences, which is in accordance with an urgency to reach the closest available shelter to escape predation. Tadpoles covered short distances relative to their body size (mean distance covered was approximately three times their body size) when escaping, in what may be related to energetic or burst swimming constraints (Dayton et al., 2005). It is also possible that fast tadpole movement gives a signal to the predator and then this signal is lost when the tadpole suddenly stops and remains motionless upon a matching background. Prey movement is usually necessary for first detection, but background matching allows the prey, once detected, to ‘freeze’ and merge again into the background before the predator can strike (Osorio & Srinivasan, 1991). On the one hand, a significantly larger number of tadpoles remained exposed after being disturbed and they increased background matching, as demonstrated here, when they stopped moving, indicating that this hypothesis is likely. On the other hand, tadpoles did not seek deeper microhabitats, maybe because choosing the deepest microhabitats in the depth gradient available in the shallow streams where they live would not render this strategy efficient to impair predator capture. Studies conducted on tadpole vision of some species show that they are myopic (Hoff et al., 1999). Their vision seems to improve during development, representing a refractive index gradient, especially during metamorphosis, probably attributable to their change from aquatic to terrestrial habitat (Mathis, Schaeffel & Howland, 1988). The range of developmental stages of B. alvarengai we used here are not supposed to include great changes in vision, as they were not very close to metamorphosis, so they could all be considered myopic (see Mathis et al., 1988). This could be another reason for their short-distance movements; they may choose microhabitats/shelters within their visual field. Otherwise, they may choose microhabitats based on cues other than visual ones, which may instead coincide with light/colour (see Hoff et al., 1999). © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 444 P. C. ETEROVICK ET AL. It is also interesting to notice that features of the tadpoles other than lightness and colour seemed to contribute to crypsis. When on sandy backgrounds, the lighter outline of the tadpole’s body seemed to help hinder detection of tadpole’s body shape (obliterative shading, sensu Stevens & Merilaita, 2009; fig. 1D), while the two light spots on the dorsum (Fig. 1A) seemed to work as ‘dazzle’ markings (distractive markings, sensu Stevens & Merilaita, 2009), which are believed to act like distractors drawing the eyes of the predator away from the body outline, preventing recognition (Stevens, 2007). Although such colour features are likely to be deceptive to visually oriented predators, their actual effects on predator avoidance remain untested for B. alvarengai tadpoles. The presence of both physical and behavioural features that enhance crypsis adds evidence to its adaptive importance for B. alvarengai tadpole survivorship. Animals that are exposed to different kinds of backgrounds may minimize predation risk by achieving a compromise cryptic strategy that confers some advantage in more than one background (Houston, Stevens & Cuthill, 2007). This may be the case for B. alvarengai tadpoles, which exhibit a combination of colours, features and behaviours that potentially increase crypsis. Besides, it has been demonstrated previously that complex backgrounds facilitate selection for cryptic mechanisms (Merilaita, 2003) and the structurally complex backgrounds of montane meadow streams are likely good for tadpole crypsis. Differences in stream characteristics could result in differences in tadpole escape opportunities and should be accounted for. For instance, the differences observed in distance covered by fleeing tadpoles in the stream sampled in December 2006 (S7) in relation to the remaining streams (S1–S6) could be because of physical differences of the stream beds. Alternatively, tadpoles may change their behaviour in response to changes in light, temperature or depending on their daily activity patterns. It is also possible that this result was influenced by different observers disturbing tadpoles, although we made an effort to standardize all sampling procedures. Differences in tadpole behaviour among streams could also be as a result of genetic differences among populations; however, there is no information available on the genetic structure of B. alvarengai populations to test this hypothesis. The differences between resting tadpoles (before disturbance) and background were significantly higher in some streams than in others, but, after disturbance, escaping tadpoles always achieved the same result of increasing similarity to background visual signals. It is possible that the differences observed before disturbance are because of different substrate availability among streams. If this is true, tadpoles were able to increase their cryptic potential, even in streams that offered more contrasting backgrounds, and to attain the same level of background matching achieved by tadpoles in streams with backgrounds more similar to their own coloration. We did not evaluate to what extent (if any) tadpoles can change their colours in order to match background. However, the time interval between the moments when we disturbed and photographed each tadpole after fleeing did not exceed 30 s, minimizing the eventual influence of colour changes, if they happen. It has been previously shown that adult individuals can change colour, but the process is slow (Tattersall et al., 2006). Thus, we interpret our results as an effect of behavioural maximization of crypsis through background selection for background matching. It is also possible that some tadpoles had been on the backgrounds where we detected them for a shorter time in some instances than in others, possibly having less time to adjust their colour accordingly. Even if it is the case, tadpoles were still able to choose a better matching background in a short time interval when disturbed, showing that, even if physiological colour change happens and aids to crypsis, the behavioural component is still important and is probably more efficient to escape imminent predator attacks. Tadpoles of B. alvarengai and other species are known to select microhabitats in a non-random way in montane meadow streams (Eterovick & Barros, 2003). Although B. alvarengai has a moderately broad spatial niche in the larval stage (Eterovick & Barros, 2003), it is not known whether preferences and niche breadth vary individually. In order to achieve better crypsis and increase survival chances, B. alvarengai tadpoles need to be able to select the most appropriate microhabitats that may be achieved through natural selection or tadpoles’ perception of cryptic potential. Tadpoles that succeed in choosing better matching backgrounds will have greater chances of surviving, whether they can actually see the substrate or use any other cue that will produce the same result. As has been shown for the salamander Plethodon cinereus, behaviours that improve an individual’s ability to escape predation may be selected for according to individual phenotype (Venesky & Anthony, 2007). Thus, an animal may be able to select backgrounds and position itself in ways that decrease predation risk through improved crypsis, as shown for grasshoppers (Eterovick et al., 1997). As prey motion is crucial for detection by predators, aquatic prey can also position themselves in ways that minimize involuntary motion caused by currents, as seen in cuttlefish (Sepia pharaonis and S. officinalis, Shohet et al., 2006). Tadpoles of B. alvarengai may be under selection to have colour types that provide crypsis through background matching in their natural habitat and behaviours that allow them to choose the appropriate background to match their colour while remaining motionless. This is an © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 CRYPSIS OF THREATENED TADPOLES interesting subject that deserves further study, including studies focusing on specific predators and their particular visual systems (see Stevens et al., 2007). Colour differences between tadpole and background after disturbance (Da*b* = 7.39) were still high enough to be noticeable to the human eye (see McCormickGoodhart & Wilhelm, 2003). However, considering that birds may have lower contrast sensitivity than humans (Ghim & Hodos, 2006), the reduction in lightness and colour contrasts relative to background achieved by escaping tadpoles may be enough to deceive predatory birds. It is important to consider that birds probably have separate sets of receptors for luminance and colour vision (Osorio & Vorobyev, 2005) and B. alvarengai tadpoles managed to decrease their contrast from the background in both these signals. Birds and many other animals have a visible spectrum spanning 300- to 700-nm range wavelength, whereas humans see within wavelengths of 400– 700 nm (Endler & Mielke, 2005). We have no information on whether B. alvarengai tadpoles and their backgrounds reflect light in wavelengths between 300 and 400 nm in order to evaluate a potential effect of these wavelengths on tadpole crypsis. In this study we showed, for the first time, that tadpoles can increase cryptic potential by selecting microhabitats that better match their lightness and colour. When disturbed, tadpoles of B. alvarengai may seek shelters, provided they are available within a short distance. Otherwise, they may remain motionless after a short escape response, on a matching background. We believe therefore that a potential trade-off in site selection exists for tadpoles, whereby they select microhabitats that offer the appropriate proximity to retreat sites; failing that potential, they will escape to microhabitats that provide appropriate background matching. Alternatively, the absence of matching backgrounds in the tadpoles’ vicinities may induce them to seek shelter. The use of defensive colorations by tadpoles is still little explored and constitutes a very interesting and complex subject. Future studies should examine the real effects on predation risk offered by different background types in order to explain tadpole behavioural adaptations. ACKNOWLEDGEMENTS We are thankful to C. M. F. Morais and D. A. Coelho for help in an initial phase of this work, during the Herpetology field course of the Programa de Pós Graduação em Zoologia de Vertebrados, PUC Minas, and to A. M. Souza for providing useful information on tadpole potential predators that occur in the Park. Permits were provided by Ibama (12813-1), and IEF (Instituto Estadual de Florestas; 085/06). Financial support was provided by FIP (Fundo de Incentivo 445 à Pesquisa) PUC Minas, and Fapemig (Fundação de Amparo à Pesquisa do Estado de Minas Gerais). A Research Productivity grant (305889/2007-9) was provided to P.C.E. by CNPq. REFERENCES Ab’Saber AN. 1977. Os domínios morfoclimáticos na América do Sul. Primeira aproximação. Geomorfologica 52: 1–21. Ayres M, Ayres Jr M, Ayres DL, Santos AS. 2005. Bioestat. Versão 4.0. Belém, Pará, Brazil: Sociedade Civil Mamirauá, MCT-CNPq. Benard MF. 2006. Survival trade-offs between two predator induced phenotypes in Pacific treefrogs (Pseudacris regilla). Ecology 87: 340–346. Bokermann WCA. 1956. Sobre uma nova especie de Hyla do estado de Minas Gerais, Brasil (Amphibia, Salientia, Hylidae). Papéis Avulsos do Departamento de Zoologia, São Paulo XII: 357–362. CIE (International Commission on Illumination). 1986. Colorimetry. Publication 15.2. 2nd edn. Vienna, Austria: CIE Central Bureau. Dayton GH, Saenz D, Baum KA, Langerhans RB, DeWill TJ. 2005. Body shape, burst speed and escape behavior of larval anurans. Oikos 111: 582–591. Drimbarean A, Whelan PF. 2001. Experiments in colour texture analysis. Pattern Recognition Letters 22: 1161–1167. Endler JA. 1978. A predator’s view of animal color patterns. Evolutionary Biology 11: 319–364. Endler JA. 2006. Disruptive and cryptic coloration. Proceedings of the Royal Society B 273: 2425–2426. Endler JA, Mielke Jr PW. 2005. Comparing entire color patterns as birds see them. Biological Journal of the Linnean Society 86: 405–431. Eterovick PC, Barata IM. 2006. Distribution of tadpoles within and among Brazilian streams: the influence of predators, habitat size and heterogeneity. Herpetologica 62: 365– 377. Eterovick PC, Barros IS. 2003. Niche occupancy in southeastern Brazilian tadpole communities in montane-meadow streams. Journal of Tropical Ecology 19: 439–448. Eterovick PC, Figueira JEC, Vasconcellos-Neto J. 1997. Cryptic coloration and choice of escape microhabitats by grasshoppers (Orthoptera: Acrididae). Biological Journal of the Linnean Society 61: 485–499. Eterovick PC, Sazima I. 2004. Anfíbios da Serra do Cipó: Amphibians from the Serra do Cipó. Belo Horizonte, Brazil: Editora PUC Minas. Ghim MM, Hodos W. 2006. Spatial contrast sensitivity of birds. Journal of Comparative Physiology A 192: 523–534. Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16: 183–190. Hoff KvS, Blaustein AR, McDiarmid RW, Altig R. 1999. Behavior. Interactions and their consequences. In: McDiarmid RW, Altig R, eds. Tadpoles. The Biology of Anuran Larvae. Chicago and London: The University of Chicago Press, 215–239. © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446 446 P. C. ETEROVICK ET AL. Houston AI, Stevens M, Cuthill IC. 2007. Animal camouflage: compromise or specialize in a 2 patch-type environment? Behavioral Ecology 18: 769–775. Hunter RS. 1948. Accuracy, precision, and stability of new photoelectric color-difference meter. Journal of the Optical Society of America 38: 1094. Kopp K, Eterovick PC. 2006. Factors influencing spatial and temporal structure of frog assemblages at ponds in southeastern Brazil. Journal of Natural History 40: 1813– 1830. Kopp K, Wachlevski M, Eterovick PC. 2006. Environmental complexity reduces tadpole predation by water bugs. Canadian Journal of Zoology 84: 136–140. Mathis U, Schaeffel F, Howland HC. 1988. Visual optics in toads (Bufo americanus). Journal of Comparative Physiology A 163: 201–213. McCollum SA, Leimberger JD. 1997. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109: 615– 621. McCollum SA, Van Buskirk J. 1996. Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50: 583–593. McCormick-Goodhart M, Wilhelm H. 2003. A new test method based on CIELAB colorimetry for evaluating the permanence of pictorial images. Wilhelm Imaging Research, Inc. Available at http://www.wilhelm-research.com/articles_ wir_additional.html. Merilaita S. 2003. Visual background complexity facilitates the evolution of camouflage. Evolution 57: 1248–1254. Merilaita S, Lind J. 2005. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proceedings of the Royal Society B 272: 665–670. Oliveira FFR, Eterovick PC. 2009. The role of river longitudinal gradients, local and regional attributes in shaping frog assemblages. Acta Oecologica 35: 727–738. Osorio D, Srinivasan MV. 1991. Camouflage by edge enhancement in animal coloration patterns and its implications for visual mechanisms. Proceedings of the Royal Society B 244: 81–85. Osorio D, Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proceedings of the Royal Society B 272: 1745–1752. Relyea RA. 2003. How prey respond to combined predators: a review and an empirical test. Ecology 84: 1827–1839. Richardson JL. 2006. Novel features of an inducible defense system in larval treefrogs (Hyla chrysoscelis). Ecology 87: 780–787. Sawaya RJ, Marques OAV, Martins M. 2008. Composição e história natural das serpentes de Cerrado de Itirapina, São Paulo, sudeste do Brasil. Biota Neotropica 8: 127– 149. Sazima I, Bokermann WCA. 1977. Anfíbios da Serra do Cipó, Minas Gerais, Brasil. 3: Observações sobre a biologia de Hyla alvarengai Bok. (Anura, Hylidae). Revista Brasileira de Biologia 37: 413–417. Shohet AJ, Baddeley RJ, Anderson JC, Kelman EJ, Osorio D. 2006. Cuttlefish responses to visual orientation of substrates, water flow and a model of motion camouflage. Journal of Experimental Biology 209: 4717–4723. Shohet A, Baddeley R, Anderson J, Osorio D. 2007. Cuttlefish camouflage: a quantitative study of patterning. Biological Journal of the Linnean Society 92: 335–345. Skelly DK, Werner EE. 1990. Behavioral and life-historical responses of larval American toads to an odonate predator. Ecology 71: 2313–2322. Stevens M. 2007. Predator perception and the interrelation between different forms of protective coloration. Proceedings of the Royal Society B 274: 1457–1464. Stevens M, Merilaita S. 2009. Animal camouflage: current issues and new perspectives. Philosophical Transactions of the Royal Society B 364: 423–427. Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. 2007. Using digital photography to study animal coloration. Biological Journal of the Linnean Society 90: 211–237. Systat Software I. 2007. Systat 12 for Windows. San Jose: Systat Software Inc. Tattersall GJ, Eterovick PC, Andrade DV. 2006. Tribute to R. G. Boutilier: Skin colour and body temperature changes in basking Bokermannohyla alvarengai (Bokermann, 1956). Journal of Experimental Biology 209: 1185– 1196. Teplitsky C, Laurila A. 2007. Flexible defense strategies: competition modifies investment in behavioral vs. morphological defenses. Ecology 88: 1641–1646. Van Buskirk J. 2000. The costs of an inducible defense in anuran larvae. Ecology 81: 2813–2821. Van Buskirk J, Anderwald P, Lüpold S, Reinhardt L, Schuler H. 2003. The lure effect, tadpole tail shape, and the target of dragonfly strikes. Journal of Herpetology 37: 420–424. Venesky MD, Anthony CD. 2007. Antipredator adaptations and predator avoidance by two color morphs of the eastern red-backed salamander, Plethodon cinereus. Herpetologica 63: 450–458. Victor R, Ugwoke LI. 1987. Preliminary studies on predation by Sphaerodema nepoides Fabricius (Heteroptera: Belostomatidae). Hydrobiologia 154: 25–32. Zaher H, Grazziotin FG, Cadle JE, Murphy RW, MouraLeite JC, Bonatto SL. 2009. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia, São Paulo 49: 115–153. © 2010 The Linnean Society of London, Biological Journal of the Linnean Society, 2010, 101, 437–446