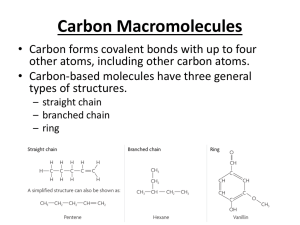
The effect of carbonyl, carboxyl and hydroxyl groups on
advertisement

摇 第 26 卷摇 第 3 期 2011 年 6 月 新摇 型摇 炭摇 材摇 料 NEW CARBON MATERIALS Vol. 26摇 No. 3 摇 Jun. 2011 Article ID:摇 1007鄄8827(2011)03鄄0224鄄05 The effect of carbonyl, carboxyl and hydroxyl groups on the capacitance of carbon nanotubes LI Li鄄xiang1 ,摇 LI Feng2 (1. Institute of Materials Electrochemistry Research, University of Science and Technology Liaoning, Anshan 114051, China; 2. Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China) Abstract:摇 Concentrated H2 SO4 颐HNO3 mixed acids, air, nitric acid and potassium permanganate were used to oxidize carbon nanotubes ( CNTs) to introduce surface functional groups ( SFGs) and the effects of the type and amount of SFGs on the electrochemical properties of CNT supercapacitors were investigated. XPS analysis shows that the mixed acid oxi鄄 dation produces carbonyl ( C詤O ) and the carboxyl ( O C詤O ) groups, the air oxidation results in hydroxyl and the smallest amount of carbonyl and carboxyl groups, and both the nitric acid and potassium permanganate treatments re鄄 sult in a moderate amount of carbonyl and carboxyl groups. It was found that the specific surface area and pore structures of the four samples are similar and carbonyl and carboxyl groups contribute the most to pseudo鄄capacitance through a Far鄄 adic reaction. In particular, the carbonyl group has a proportional relationship to the capacitance of CNTs. However, the hydroxyl group does not lead to an obvious increase of pseudo鄄capacitance, but can increase the electric double layer ca鄄 pacitance. The carbonyl and the carboxyl groups are advantageous for fast Faradic reactions to introduce pseudo鄄capaci鄄 tance, owing to their lower charge transfer resistance than that of the hydroxyl group. Keywords:摇 Carbon nanotubes; Carbonyl; Carboxyl; Hydroxyl; Pseudo鄄capacitance CLC number:摇 TB 383 Document code:摇 A 1摇 Introduction Carbon nanotubes ( CNTs ) are typical one鄄di鄄 mensional carbon materials with a unique pore struc鄄 ture and have excellent electronic, mechanical and chemical properties [1鄄5] , which along with their high surface area and large accessible mesopores make them suitable for transport of electrolyte ions. There鄄 fore, CNTs are good candidates for electrode materi鄄 als of electric double layer capacitors ( ECs ) [6鄄8] . However, one impediment to the potential application of CNTs as electrode materials of ECs is their poor hydrophility, especially when aqueous electrolytes are used. Therefore, the specific capacitance of CNTs is low. In order to improve the hydrophility of CNTs for further application, a variety of chemical treatments or purification processes are used. Among them, the chemical treatments using oxidation agents such as acid, mixed acid and air are commonly used owing to their simplicity and efficiency in removing impurities ( such as metallic catalysts) and producing oxygen鄄 containing surface functional groups ( SFGs) to en鄄 hance their hydrophility. Moreover, it is found that these chemical treatments can result in an increase of specific capacitance of CNT electrode because some of SFGs can provide pseudo鄄capacitance by reacting with electrolyte ions [9鄄11] , in addition to the electrical double layer capacitance ( EDLC) formed by charge separation on the interface between the electrode and the electrolyte. 摇 摇 It has been known that the SFGs can obviously affect the capacitance of CNTs. Generally, the higher the amount of SFGs, the higher the capacitance. However, SFGs from chemical treatment of CNTs of鄄 ten consist of the carbonyl, carboxyl and hydroxyl groups [12] . The influence of these different kinds of SFGs on the capacitance of CNTs is still not fully studied. 摇 摇 In order to investigate the effects of different kinds of SFGs on the capacitance of CNTs, four typi鄄 摇 Received date:2011鄄03鄄08;摇 Revised date:2011鄄06鄄02 摇 Foundation item: National Science Foundation of China (50328204, 50472084 and 50632040) , Natural Science Foundation of Liaoning Province of China (20061078) and Foundation of Liaoning Education Committee of China ( L2010197) . 摇 Corresponding author: LI Feng, Professor. Fax: +86鄄24鄄23971471, E鄄mail: fli@ imr. ac. cn 摇 Author introduction: LI Li鄄xiang(1972-) , female, Dr. , working on synthesis and electrochemistry of advanced carbons. E鄄mail: lxli2005@ 126. com 摇 English edition available online ScienceDirect ( http:蛐蛐www. sciencedirect. com蛐science蛐journal蛐18725805 ) . 摇 DOI: 10. 1016 / S1872鄄5805(11)60078鄄4 第3 期 LI Li鄄xiang et al: The effect of carbonyl, carboxyl and hydroxyl groups on the capacitance of … cal treatment methods with different oxidation abilities were used to control the amount and composition of SFGs on the CNTs. They were the mixed acid oxida鄄 tion with nitric acid and vitriol, nitric acid oxidation, potassium permanganate oxidation and air oxida鄄 tion [13鄄18] . Wet chemical treatments such as acid and potassium permanganate oxidation can produce SFGs containing more carboxyl and carbonyl groups. Espe鄄 cially, acid oxidation produces more acidic carboxylic groups [19鄄20] and the gaseous oxidation generally re鄄 sults in more hydroxyl groups [21鄄22] . Furthermore, the content of the SFGs on CNTs is dependent on the oxi鄄 dation ability of the treating agents. After the treat鄄 ment, the SFGs on CNTs were characterized and their effects on the specific capacitance of CNT electrode are compared. It was found that the SFGs of the hy鄄 droxyl ( C—O) , carbonyl ( C詤O ) , and carboxyl ( O C詤O ) groups have different contributions to capacitance. 2摇 Experimental 2. 1摇 Chemical treatments Multi鄄walled CNTs ( MCNTs) were synthesized by catalytic decomposition of benzene at 1423 K using iron compounds as catalysts [23] . The four types of pretreatment are widely used, whether it is purifica鄄 tion or functionalization. Therefore, they were used to control the SFGs of MCNTs in our study. The as鄄 prepared MCNT sample was heated in nitric acid ( mass fraction is 68% ) mixed with vitriol ( mass fraction is 98% ) at 398 K in oil bath for 0. 5 h to ob鄄 tain the mixed acid oxidized sample [13] , which is de鄄 noted as MCNT鄄1. The as鄄prepared MCNT sample was dispersed in 68% HNO3 and heated at 413 K for 4. 5 h in oil bath to obtain the nitric acid oxidized sam鄄 ple denoted as MCNT鄄2 [14] . The potassium perman鄄 ganate oxidized sample was denoted as MCNT鄄3 [15] . The as鄄prepared MCNT sample was heated at 893 K for 25 min in air, and then immersed in a 1 颐1 ( vol. / vol. ) solution of deionized water and concentrated hydrochloric acid to obtain the air oxidized sample de鄄 noted as MCNT鄄4 [16鄄18] . 2. 2摇 Characterization The morphologies of the samples were character鄄 ized by a field鄄emission scanning electron spectro鄄 scope ( SEM, JEOL JSM鄄6301F) . The pore size dis鄄 tributions ( PSDs ) and the Brunauer鄄Emmett鄄Teller ( BET) specific surface area ( SSA) were measured with a volumetric adsorption apparatus ( ASAP2010M) from nitrogen adsorption isotherms at ·225摇 · 77 K. The chemical compositions and functional group distributions of the CNTs were analyzed using X鄄ray photoelectron spectroscopy ( XPS) . The XPS was recorded with an ESCALAB250 spectrometer and Mg K琢 radiation ( h淄 = 1253. 6 eV) . The deconvolu鄄 tion of the spectra was performed using a non鄄linear least squares fitting program with a symmetric Gaussi鄄 an function. 2. 3摇 Electrochemical measurements The samples were spread onto a 10鄄mm鄄diameter nickel foam followed by pressing under 10 MPa as the working electrode ( WE) . The mass of MCNTs on each WE was about 10 mg. Two WEs covered with the same amount of samples were separated by a ny鄄 lon paper and assembled in a sandwich type cell. The electrolyte was a 6 mol·L -1 KOH aqueous solution. The voltage window for the two electrode capacitor was 0 to 1 V. The reported gravimetric capacitance was normalized to the mass of MCNTs on a single WE base, which was obtained on Arbin BT2000 at a current of 1mA. Cyclic voltammograms ( CV) of the four samples were measured in a three鄄electrode cell by using a Solartron electrochemistry test system. A saturated calomel electrode ( SCE) was used as a ref鄄 erence electrode and a Pt plate was used as a counter electrode. 3摇 Results and discussion 3. 1摇 The morphologies and PSDs of MCNTs The SEM images of four MCNT samples are shown in Fig. 1, which show similar length / diameter ratios and no obvious difference in the morphologies of samples after four different treatments. The BET SSAs and pore volumes of four samples are listed in Table 1. It can be seen that four treatment methods do not lead to significant differences in the SSA, which 2 are 33, 25, 23 and 21 m· g -1 for MCNT鄄1, MCNT鄄2, MCNT鄄3 and MCNT鄄4, respectively. The small me鄄 sopores (2鄄5 nm in pore diameter) are especially use鄄 ful to the EDLC using aqueous electrolyte, because they are suitable for ion transport and storage, and thus have a great contribution to EDLC [24] . From Ta鄄 ble1, it can be seen that MCNT鄄1, 2 and 4 have very close values for mesoporous volumes, but MCNT鄄3 has a relatively low mesopore volume. On the basis of the fact that the SSA and PSDs of CNTs treated by the four methods are similar, the differences in the specific capacitance of CNTs can be used to investi鄄 gate the influence of SFGs. ·摇 226· 第 26 卷 新摇 型摇 炭摇 材摇 料 Fig. 1摇 SEM images of four samples: ( a) MCNT鄄1, ( b) MCNT鄄2, ( c) MCNT鄄3 and ( d) MCNT鄄4 Table 1摇 BET surface areas and pore structure parameters of the modified MCNTs Characterization 2 -1 S BET / m· g 3 -1 v meso a / ( cm· g ) ´10 -2 MCNT鄄1 MCNT鄄2 MCNT鄄3 MCNT鄄4 33 2. 25 25 2. 03 23 1. 08 21 2. 33 Notation: Surface area ( S BET ) and mesopore volume ( v meso a ) were calculated from the adsorption鄄desorption isotherm of N2 at 77 K by multi鄄point BET and BJH equation, respectively. 3. 2摇 The contribution of SFGs to the capacitance of MCNTs Fig. 2 presents the ratios of surface functional groups obtained by Gauss curve fitting of the peaks from the original XPS spectra. MCNT鄄1 has the lar鄄 gest proportion of oxygen鄄containing SFGs of 27. 8% , the highest content of carbonyl and carboxyl groups and the same amount of hydroxyl group as MCNT鄄2 and MCNT鄄3, while the other samples have almost the same quantity of oxygen鄄containing SFGs from 18% to 18. 9% . MCNT鄄2 and MCNT鄄3 contain more carbonyl and carboxyl groups, especially car鄄 bonyl, than MCNT鄄4. However, MCNT鄄4 has the largest amount of hydroxyl group among the four samples. The specific capacitances of four samples are 35, 25, 20 and 8 F·g -1 for MCNT鄄1, MCNT鄄2, MCNT鄄3 and MCNT鄄4, respectively. On the basis of similar SSA and pore structure of samples, it can be deduced that most of the capacitance of MCNT鄄1 should be contributed by the carbonyl and the carbox鄄 yl groups, because MCNT鄄1 has much more carbonyl and carboxyl groups, especially carbonyl, than the other samples. Furthermore, it is also worth noting that MCNT鄄2, MCNT鄄3 and MCNT鄄4 have almost the same amount of SFGs but the specific capacitance of MCNT鄄4 is much lower than those of MCNT鄄2 and MCNT鄄3. It can be found, by comparing the kinds of SFGs of these three samples, that MCNT鄄4 contains the largest amount of hydroxyl group but the lowest carbonyl group, suggesting that carbonyl group is more effective in increasing the capacitance of MC鄄 NTs. In order to analyze the relationship between the capacitance and SFGs, the specific capacitances of the four samples are plotted as a function of the content of different SFGs. It can be observed that the capaci鄄 tance of MCNTs is proportional to the amount of car鄄 bonyl as shown in Fig. 3, but such relationship is not found for the other two SFGs. It is also noted that the capacitance of MCNT鄄3 is slightly lower than that of MCNT鄄2 despite their similar composition of SFGs and SSA, which can be attributed to the obviously low mesopore volume of MCNT鄄3. Fig. 2摇 The groups爷 ratio of samples. Sum stands for the sum of functional groups Fig. 3摇 Relationship between specific capacitance and the proportion of carbonyl Fig. 4 shows the cyclic voltammogram ( CV) of the four samples in 6 mol·L -1 KOH electrolyte. It can be noted that the strong electrochemical polarization occurs in the potential range of 0. 4 to -0. 2 V for 第3 期 LI Li鄄xiang et al: The effect of carbonyl, carboxyl and hydroxyl groups on the capacitance of … MCNT鄄1, MCNT鄄2 and MCNT鄄3 besides redox peaks at about 0 V. This might be caused by a steric hindrance for Faradic reaction from some groups such as carboxyl [25] . However, the shape of CV for MC鄄 NT鄄4 is very close to a rectangular and only weak po鄄 larization can be observed, which suggest that the ca鄄 pacitance of MCNT鄄4 is mainly contributed by EDLC. According to the characteristics of CV and the composition of SFGs for the four samples, it can be deduced that the contribution of carboxyl and carbonyl groups to CNTs is pseudo鄄capacitance. In a basic electrolyte, carbonyl and carboxyl groups can react with the electrolyte ions via the following path鄄 way [12,26] : > C詤O + OH - = —COOH, —COOH + OH - = —COO - +H2 O. Fig. 4摇 CV curves of four samples In addition, hydroxyl groups may enhance EDLC because of an improvement of hydrophility of CNTs [25] . To further analyze the influence of differ鄄 ent SFGs on the electrochemical property of CNTs, the electrochemical impedance spectrum of the four samples was measured as shown in Fig. 5. All spectra include semicircles in the high鄄frequency region and a straight line in the low鄄frequency region, which repre鄄 sents the typical characteristics of a supercapacitor. The semicircle in high frequency reflects the resistance of charge transfer ( R ct ) for Faradic reaction and the diameter of the semicircle is proportional to R ct . Fig. 5 illustrates that R ct decreases with the increase on the amount of carbonyl and carboxyl groups. It sug鄄 gests that these two SFGs enhance the charge transfer for the fast faradic reaction, which is possibly attribu鄄 ted to their high electron density from the double bonds between carbon and oxygen atoms. It is also noted that SFGs for MCNT鄄2 and MCNT鄄3 have al鄄 most the same composition, but MCNT鄄3 has the lar鄄 ger R ct , which can be attributed to the lower ion transport rate of MCNTs鄄3 due to its lower mesopore volume than that of MCNT鄄2. ·227摇 · Fig. 5摇 Electrochemical impedance spectra of four samples 4摇 Conclusions The influence of SFGs on the capacitance of MCNTs is investigated by using different oxidation treatments for CNTs to control the content and com鄄 position of SFGs. It is found that the carbonyl and carboxyl groups can contribute to the capacitance of CNTs electrode mostly via Faradic reactions and the content of carbonyl is proportional to the capacitance. The CNT treated by the mixed acid has the highest ca鄄 pacitance because of the largest amount of carbonyl and carboxyl groups. The hydroxyl group does not contribute obviously to pseudo鄄capacitance, but en鄄 hances electronic double layer capacitance by an in鄄 creased wettability. The MCNTs treated by air oxida鄄 tion have the lowest capacitance despite the largest amount of hydroxyl group, as a result of the least amount of carbonyl and carboxyl groups. Moreover, the carbonyl and carboxyl groups have lower charge transfer resistance and thus are advantageous to fast faradic reactions to introduce pseudo鄄capacitance. References [1] 摇 Liu T, Sreekumar T V, Kumar S, et al. SWNT / PAN composite film鄄based supercapacitors [ J] . Carbon, 2003, 41: 2427鄄2451. [2] 摇 Park J H, Choi J H, Moon J S, et al. Simple approach for the fabrication of carbon nanotube field emitter using conducting paste[ J] . Carbon, 2005, 43: 698鄄703. [3] 摇 Sayago I, Terrado E, Lafuente E, et al. Hydrogen sensors based on carbon nanotubes thin films[ J] . Synth Met, 2005, 148: 15鄄 19. [4] 摇 Zhao Q, Frogley M D, Wagner H D. Direction sensitive stress measurements with carbon nanotube sensors [ J ] . Polym Adv Technol, 2002, 13: 759鄄764. [5] 摇 Somani P R, Somani S P, Flahaut E, et al. Improving the pho鄄 tovoltaic response of a poly( 3鄄octylthiophene) / n鄄Si heterojunc鄄 tion by incorporating double鄄walled carbon nanotubes[ J] . Nano鄄 technology, 2007, 18: 185708鄄1鄄185708鄄5. [6] 摇 Frackowiak E, Jurewicz E, Delpeux S, et al. Nanotubular mate鄄 rials for supercapacitors [ J] . J Power Sources, 2001, 97鄄98: 822鄄825. ·摇 228· 第 26 卷 新摇 型摇 炭摇 材摇 料 [7] 摇 Ma R Z, Liang J, Wei B Q, et al. Study of electrochemical ca鄄 pacitors utilizing carbon nanotube electrodes [ J ] . J Power Sources, 1999, 84: 126鄄131. [8 ] 摇 Emmenegger C, Mauron P, Sudan P, et al. Investigation of electrochemical double鄄layer capacitors electrodes based on car鄄 bon nanotubes and activated carbon materials [ J ] . J Power Sources, 2003, 124: 321鄄329. [9] 摇 Seo M K, Park S J. Electrochemical characteristics of activated carbon nanofiber electrodes for supercapacitors [ J ] . Mater Sci Eng B, 2009, 164: 106鄄111. [10] 摇 Bleda鄄Martinez M J, Macia鄄Agullo J A, Lozano鄄Castello D, et al. Role of surface chemistry on electric double layer capaci鄄 tance of carbon materials[ J] . Carbon, 2005, 43: 2677鄄2684. [11] 摇 Ruiz V, Blanco C, Raymundo鄄Pinero E, et al. Effects of ther鄄 mal treatment of activated carbon on the electrochemical behav鄄 iour in supercapacitors[ J] . 2007, 52: 4969鄄4973. [12] 摇 WANG Da鄄wei , LI Feng, LIU Min, et al. Improved capaci鄄 tance of SBA鄄15 templated mesoporous carbons after modifica鄄 method of opening and filling carbon nanotubes [ J] . Nature, 1994, 372: 159鄄162. [18] 摇 Kosaka M, Ebbesen T W, Hiura H, et al. Electron鄄spin鄄reso鄄 nance of carbon nanotubes[ J] . Chem Phys Lett, 1994, 225: 161鄄164. [19] 摇 H Ago, Kugler T, Cacialli F, et al. Work functions and surface functional groups of multiwall carbon nanotubes [ J ] . J Phys Chem B 1999, 103: 8116鄄21. [20] 摇 Jung A, Graupner R, Ley L, et al. Quantitative determination of oxidative defects on single walled carbon nanotubes [ J ] . Phys Stat Sol ( b) 2006, 243: 3217鄄3220. [21] 摇 Hsieh C, Teng H. Influence of oxygen treatment on electric double鄄layer capacitance of activated carbon fabrics [ J] . Car鄄 bon, 2002, 40: 667鄄674. [22] 摇 Hsieh C, Chen W, Cheng Y S. Influence of oxidation level on capacitance of electrochemical capacitors fabricated with carbon nanotube / carbon paper composites [ J ] . Electrochimica Acta, 2010, 55: 5294鄄5300. tion with nitric acid oxidation [ J ] . New Carbon Materials, [23] 摇 侯鹏翔. 多壁纳米碳管的大量制备、提纯及其储氢性能研究 ( 王大伟,李摇 峰,刘摇 敏,等. 硝酸氧化改性 SBA鄄15 模板合 ( Hou Peng鄄xiang. Large鄄scale synthesis, purification and hy鄄 2007, 24: 307鄄314. 成的中孔炭电容性能研究[ J] . 新型炭材料,2007, 22 (4 ) : 307鄄314. ) [13] 摇 Liu J, Rinzler A G, Dai H J, et al. Fullerene pipes[ J] . Sci鄄 ence, 1998, 280: 1253鄄1256. [14] 摇 Dujardin E, Ebbesen T W, Krishnan A, et al. Purification of single鄄shell nanotubes[ J] . Adv Mater, 1998, 10: 611鄄613. [15] 摇 Hiura H, Ebbesen T W, Tanigaki K. Opening and purification of carbon nanotubes in high yields[ J] . Adv Mater, 1995, 7: 275鄄276. [16] 摇 Ajayan P M, Ebbesen T W. Opening carbon nanotubes with oxygen and implications for filling [ J] . Nature, 1993, 362: 522鄄525. [17] 摇 Tsang S C, Chen Y K, Harris P J F, et al. A simple chemical [ D] . 沈阳: 中国科学院金属研究所, 2003. drogen storage capacity investigations of multi鄄walled carbon nanotubes[ D] . Shenyang: Institute of Metal Research, Chinese Academy of Sciences, 2003. ) [24] 摇 Ciaz J, Paolicelli G, Ferrer S, et al. Separation of the sp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films[ J] . Phys Rev B, 1996, 53: 8064鄄8069. [25] 摇 Oda H, Yamashit A, Minour S, et al. Modification of the oxy鄄 gen鄄containing functional group on activated carbon fiber in e鄄 lectrodes of an electric double鄄layer capacitor [ J ] . J Power Sources, 2006, 158: 1510鄄1516. [26] 摇 Nian U R, Teng H. Influence of surface oxides on the imped鄄 ance behavior of carbon based electrochemical capacitors[ J] . J Electroanalytical Chem, 2003, 540: 119鄄127. 羰基、羧基和羟基表面官能团 对碳纳米管电容量的影响 李莉香1 ,摇 李摇 峰2 (1. 辽宁科技大学 材料电化学研究所,辽宁 鞍山 114051; 2. 中国科学院金属研究所 沈阳材料科学国家( 联合) 实验室, 辽宁 沈阳 110016) 摘摇 要:摇 分别采用混酸、空气、硝酸和高锰酸钾对碳纳米管进行氧化处理,以在其表面引入官能团,进而研究了表 面官能团对碳纳米管电化学性能的影响。 X鄄射线光电子谱分析表明:混酸氧化处理引入的官能团主要为羰基和羧 基;空气氧化使碳纳米管表面链接较多的羟基,但羰基和羧基的含量最少;而硝酸处理和高锰酸钾处理引入了中等 数量的羰基和羧基。 经四种处理方法所得碳纳米管具有相近的比表面积和孔结构。 通过比较它们的比电容发现: 羰基和羧基贡献了最多的准电容,尤其羰基含量与碳纳米管的电容量呈正比关系;而羟基主要增强了双层电容,并 未引入明显的准法拉第容量。 由于羰基和羧基比羟基具有更低的电荷传递电阻,有利于快速的法拉第反应,从而 引入准电容。 关键词:摇 碳纳米管;羰基;羧基;羟基;准电容 基金项目:国家自然科学基金(50328204, 50472084, 50632040) ,辽宁省自然科学基金(20061078) 和辽宁省教育厅基金( L2010197) . 通讯作者:李摇 峰. E鄄mail: fli@ imr. ac. cn 作者介绍:李莉香(1972-) ,女,辽宁人,博士,研究方向:先进炭材料合成及电化学应用. E鄄mail: lxli2005@ 126. com