Carbohydrates - Faculty Pages
advertisement
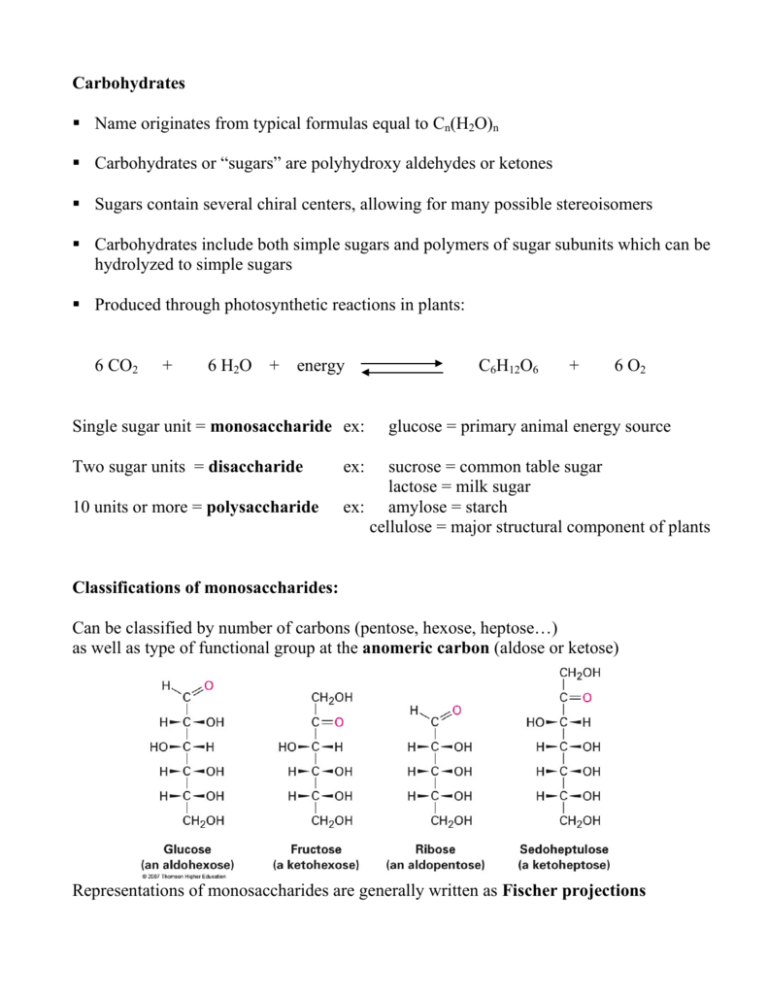
Carbohydrates Name originates from typical formulas equal to Cn(H2O)n Carbohydrates or “sugars” are polyhydroxy aldehydes or ketones Sugars contain several chiral centers, allowing for many possible stereoisomers Carbohydrates include both simple sugars and polymers of sugar subunits which can be hydrolyzed to simple sugars Produced through photosynthetic reactions in plants: 6 CO2 + 6 H2O + energy Single sugar unit = monosaccharide ex: Two sugar units = disaccharide 10 units or more = polysaccharide C6H12O6 + 6 O2 glucose = primary animal energy source ex: sucrose = common table sugar lactose = milk sugar ex: amylose = starch cellulose = major structural component of plants Classifications of monosaccharides: Can be classified by number of carbons (pentose, hexose, heptose…) as well as type of functional group at the anomeric carbon (aldose or ketose) Representations of monosaccharides are generally written as Fischer projections Drawing Fischer Projections: Fisher projections are used to represent molecules with chiral centers in two dimensions. They are especially useful for molecules that contain two or more chiral centers. • • • • Orient the molecule so that two groups point toward you and two point away Each chiral carbon is represented as the center of two crossed lines Horizontal lines represent bonds coming out of the plane of the page Vertical lines represent bonds going back behind the plane of the page R and S configurations are best determined by rotating the molecule in such a way that the lowest priority group (usually H) is at the top or the bottom and applying the clockwise/counterclockwise turn rule: Example: S-alanine A Fischer projection can be rotated by 180o and still remain the same configuration. However, rotating it by 90o in either direction gives the opposite configuration Molecules with several chiral centers are drawn so that they run vertically: Stereochemistry, nomenclature and D & L notation Sugars that are enantiomers have the same name Carbohydrates (and amino acids) are given D or L notations which are assigned by comparing the structures to D and L-glyceraldehyde: Natural sugars are generally the D – isomers (+) and (-) designations are determined experimentally based on rotation Diastereomers of the sugars For any sugar, the number of possible diastereomers = 2n where n = # of chiral centers There are 8 D-aldohexose diastereomers; D-glucose, D-galactose and D-mannose being the most common in nature D-ribose is the most common aldopentose, a component of RNA Diastereomers which differ in configuration only at one C are called “epimers” Ketoses with a given number of C have half as many isomers as the aldoses due to internal C = O (one less chiral center) Behavior of sugars in solution: ring formation In aqueous solution, a very small % of monosaccharide molecules is found in the openchain form. Rather, they form cyclic hemiacetals (aldoses) or hemiketals (ketoses). • Because the anomeric carbonyl carbon becomes a chiral center in the hemiacetal, two more isomeric forms exist for each sugar: anomers • The α and β forms are in equilibrium with open-chain form and can interconvert. • The resulting change in optical rotation is called mutarotation • Both aldoses and ketoses prefer to form 5 or 6-member rings (furanoses and pyranoses) Drawing the cyclic forms: Haworth projections 1) The O is shown at the back right, with the anomeric C at right-most position 2) The remaining C in the ring are drawn clockwise. For aldohexoses, C5 attaches to O to make the 6-member ring, so C6 is shown above the ring. HO CH2 OH O OH H H OH H OH H 3) Groups to the right are drawn pointing down; left-hand groups point up 4) α-anomers have anomeric OH pointing DOWN, β = pointing UP Why is glucose so common in nature? Why would the β-anomer form more often? Haworth projections are somewhat misleading; the ring is represented as being flat In solution, pyranose rings take the chair conformation: In β-D-glucose, all the large groups will fall in the equatorial position at the same time; thus decreasing steric strain and increasing stability. Other aldohexose diastereomers of glucose will have at least one OH group in axial position: Ketoses: the anomeric C is not C1 but C2. Therefore, the α-anomer will have a CH2OH pointing up from C2 (see fructose below): Reduction and Oxidation Reactions of Monosaccharides Since alcohols, aldehydes & ketones easily undergo oxidation & reduction reactions, it's not surprising that many such reactions take place biologically & synthetically Reactions involving the C=O only occur while the sugar is in its open chain form 1. Reactions with reducing agents: The carbonyl group of aldoses & ketoses is readily reduced to an alcohol, forming alditols Reagents: NaBH4, H2 with Pd/C 2. Oxidations and "reducing sugars" Oxidations of the carbonyl group of aldoses are common both in diagnostic classification reactions and biological reactions. Aldoses oxidize more readily than ketoses. Depending on strength of ox. agent, aldonic or aldaric acids may form: Ketoses can be oxidized under basic conditions because they can isomerize to an aldose through an enol intermediate: Classification tests: Identification of a reducing sugar makes use of oxidizing agents As long as a sugar has a free aldehyde group it behaves as a reducing sugar. Ex: Barfoed's test = copper (II) acetate Benedict’s reagent = copper (II) citrate Tollens' test = silver (I) ammonia complex + Cu(CH3COO)2 D-glucose Barfoed's Reagent + Cu2O + CH3COOH D-gluconic acid Copper(I) oxide Biological example of sugar oxidation: In glycolysis, which is the metabolic conversion of glucose to pyruvate, NAD+ functions as the oxidizing agent in converting glyceraldehyde-3-phosphate to its oxidized form: 2 NAD +, 2 P 2 NADH Acetylation: an esterification Sugars can be converted into acetyl esters to make them easier to handle Ether formation: xs CH3I, AgNO3 Imine formation: Osazones and sugar identification An osazone is a solid derivative of a sugar formed through reaction with phenylhydrazine (similar to the 2,4-DNPH for aldehydes & ketones) Used to identify unknown sugars An imine forms with the C = O carbon and one adjacent carbon (C2 or C1) C-2 epimers form the same osazone structure Glucose and fructose form the same osazone because they have identical configurations from C3 - C6: Chain-lengthening and chain shortening: making sugars bigger or smaller The modified Kiliani -Fischer synthesis begins with nucleophilic addition of cyanide, followed by reduction with hydrogen, then hydrolysis to form a longer aldose A new chiral center is generated, so you get two epimers C HC HO O H HCN N C HO H H HO H HO N OH H H2 Pd H OH H OH H OH H OH H OH H OH H 2 C OH H 2 C OH HC O H 2 C OH H3O+ HC O HO H H HO H HO OH H H OH H OH H OH H OH H 2 C OH H 2 C OH The Wohl degradation shortens an aldose by one carbon using the opposite process How monosaccharides link together: glycosides and polysaccharides In disaccharides or polysaccharides, sugar units are linked together through formation of glycosidic bonds Links two sugar units through acetal or ketal formation The anomeric OH of one sugar links to an OH group on another sugar unit Linkage of sugars to other sugars or other types of molecules = glycosidic linkage OH OH O HO OH CH 2 O HO O OH O OH HO Mechanism of Glycoside Formation: Quercetin galactoside, an antioxidant found in berries Types of glycosidic linkages in di- and polysaccharides In nature, enzymes catalyze reactions between specific anomers. α-1,4: The α-anomer of one sugar forms a linkage through C1 to C4 of the next unit Ex: maltose β-1,4: The β-anomer forms the linkage through C1 to C4 of next unit Ex: cellobiose lactose When a disaccharide linkage does not involve the anomeric C of one of the units, that C remains in hemiacetal linkage and can convert to open (aldehyde) form in solution. These disaccharides are "reducing sugars" An acetal linkage at the anomeric carbon prevents that ring from opening in solution. Sugars that are bonded in such a way that the hemiacetal or hemiketal is converted to an acetal or ketal do not react like open-chain sugars: these are “non-reducing” sugars. Recall that reducing sugars react with oxidizing agents…the sugar gets oxidized and the agent gets reduced. Maltose and lactose are reducing sugars whereas sucrose is not In sucrose, the two anomeric carbons of glucose and fructose link to form the ketal α1, β2: C1 of the α-anomer of glucose bonds to C2 of the β-anomer of fructose: Some conditions related to disaccharide metabolism: Lactose intolerance: The enzyme required to hydrolyze the β-1,4 linkage of lactose is lactase; this enzyme is missing (or has disappeared over time) Galactosemia: The enzyme required to convert galactose to glucose for use in the human body is missing, causing buildup of galactose in the bloodstream, leading to brain malfunction. Periodontal disease/gingivitis Plaque buildup results from conversion of dietary sucrose into the polysaccharide dextran (α-1,3 and α-1,6-linkages of glucose) - process is accelerated by presence of bacteria Polysaccharides: Polymers of monosaccharide units Amylose: A component of starch (glucose storage for plants) Composed of glucose units in α-1,4-linkages 1000-2000 glucoses in chains that form a spiral or helical 3-D structure Can be digested by humans in enzymatic reactions similar to acid-catalyzed hydrolysis of the glycosidic linkages Amylopectin: Major component of starch May contain up to a million glucose units Glucose units connected in α-1,4-linkages with α-1,6linkages leading to branches Glycogen Storage form of glucose in the human body, found mainly in liver & muscles Structure is similar to amylopectin but with more frequent, shorter branches Hydrolyzed to glucose when energy is required Cellulose Structural component of plants, found in cell walls Up to 3000 glucose units per molecule Not digestible by humans Glycosidic linkages are all β-1,4 linkages, leads to straight chains, no helix Chains line up side by side to form rigid layers or fibers held together by hydrogen bonding between the many OH groups Paper, wood, cotton, linen all come from cellulose; the fibers will not dissolve in water but are extremely absorbent due to hydrogen bonding! Rayon & cellophane are “reconstituted” cellulose; the cellulose is broken down to a viscous liquid and drawn into fibers or sheets Chitin Structural component of crustacean shells & insect exoskeletons Composed of modified glucose; C2 OH group is converted to N-acetylamino group Glycosidic linkages are β-1,4 like in cellulose. A modified polysaccharide: heparin An anticoagulant found in arterial wall cells Made up of several different subunits having amino groups (glucosamines)and sulfonate groups Further examples of sugars in nature DNA & RNA: nucleoside and nucleotide structure The aldopentoses ribose and 2’-deoxyribose are found in the nucleotides that make up RNA and DNA The anomeric C is bonded to the nitrogen of a heterocyclic amine to form a nucleoside The C3’ forms a phosphate ester with phosphoric acid to make nucleotides The phosphate group can also form a phosphate ester to C5’ on another nucleotide, which is how a nucleic acid chain is arranged: Glycosides of lipids and proteins are quite common in nature Sphingolipids – cerebrosides Glycoproteins—includes collagen & immunoglobulins Why is carbohydrate structure on glycoproteins critical? Cell surface glycoproteins often act as receptors and recognition sites for hormones, toxins, viruses, antibiotics (they must be able to bind to work) Example: A, B, AB & O blood types Blood type is associated with carbohydrate structure on cell surface A, B & O type all have L-fucose and N-acetyl-D-glucosamine (an amino sugar) Type A and B blood cells have one sugar attached which is different Cells do not “recognize” the other sugar so the immune system makes antibodies against it As a result, only type AB persons can receive either A or B blood