Pain Pathways and Patterning
advertisement

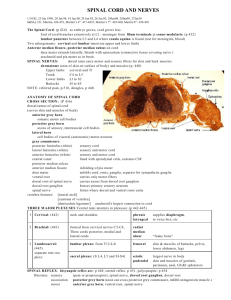
Pain Pathophysiology and Pathways: a foundation Emily DeSantis, D.O. Bassett Healthcare Network, Cooperstown, NY AOCPMR Mid-year Meeting April 20, 2012 Disclosures: No disclosures to report “That really hurts” http://youtube.com/watch?v=_OBlgSz8sSM Pre-Test Questions 1. Which proteins activate primary afferent nociceptor (PAN) nerve endings? A. Bradykinins B. Histamines C. Prostaglandins D. Serotonin E. Substance P F. All of the above Pre-Test Questions 1. Which proteins activate primary afferent nociceptor (PAN) nerve endings? A. Bradykinins B. Histamines C. Prostaglandins D. Serotonin E. Substance P F. All of the above Pre-Test Questions 2. True or False: Neuropeptides like Substance P are released from PAN terminals into the dorsal horn of the spinal cord, AND from (PAN) nerve endings in the periphery. Pre-Test Questions 2. True or False: Neuropeptides like Substance P are released from PAN terminals into the dorsal horn of the spinal cord, AND from (PAN) nerve endings in the periphery. TRUE Pre-Test Questions 3. The development of pathologic pain states causing hyperalgesia and allodynia involves which of the following theorized processes? A. Spinal sensitization B. Loss of inhibitory interneurons from excitotoxicity C. Perpetuation of the inflammatory response/peripheral nociception cascade D. Activation of “silent nociceptors” E. Deficits in the “inflammatory reflex” including the cholinergic anti-inflammatory pathway F. All of the above Pre-Test Questions 3. The development of pathologic pain states causing hyperalgesia and allodynia involves which of the following theorized processes? A. Spinal sensitization B. Loss of inhibitory interneurons from excitotoxicity C. Perpetuation of the inflammatory response/peripheral nociception cascade D. Activation of “silent nociceptors” E. Deficits in the “inflammatory reflex” including the cholinergic anti-inflammatory pathway F. All of the above Objectives Review key neurotransmitters and neuroanatomical structures involved in peripheral pain Discuss proposed hypotheses of pain mechanisms including the Inflammatory Cascade, Dorsal Root Reflexes, Gate Control Theory, and Spinal Facilitation Briefly review treatment approaches designed to modify what we understand of these “Ascending” and “Descending” pathways Outline Review nomenclature Peripheral pain pathways Spinal cord facilitation gate and pathophysiology theory, wind-up, and plasticity Central pain pathways ascending and descending modulators Treatment strategies What is Nociception? Nociception is a mechanical or chemical event, originating in peripheral tissues. * This includes bone, intervertebral discs, muscles, vasculature, and viscera as well as cutaneous tissue Nociception is (arguably) consistent between individuals. What is Pain? The International Association for the Study of Pain Subcommittee on Taxonomy defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” (Merskey & Bogduk 1994) Pain is a perception of the activity of nociception in the spinal cord, brainstem, and cortex. Pain varies with cognitive and emotional states. Perception of pain can be modulated. Types of Pain Acute pain is a normal condition in which “pain is a symptom of tissue injury.” (Bloodworth et al in Braddom 2004) Purpose: Activates the arousal system to form a protective action or response (ex. wound healing) to nociceptive event Result: Changes in the body Muscle stiffening and altered ROM Vasodynamic changes, shifting of circulatory compartments Local release of pro-inflammatory compounds Metabolic alterations favoring glucose production New adaptive state, “ALLOSTASIS” the maintenance of stability through change Types of Pain Chronic pain is “an abnormal condition…in which pain and pain behavior become the primary disease processes.” (Bloodworth et al in Braddom 2004) Failure to re-establish homeostasis Consequences: repetitive nociceptive input on plastic neurons → neuromodulation/spinal facilitation → hyperalgesia (↑gain), allodynia (↓threshold), analgesia (↓output) sustained allostasis → slow, progressive organ system damage Types of Pain Allodynia refers to pain evoked by a non-painful stimuli (ie. touch) As acute pain processes progress, “a threshold...is lowered to the extent that body temperature and the pressure of edema are adequate stimuli to result in spontaneous pain.” (Sorkin 1999) Hyperalgesia refers to an increased pain or heightened response evoked by a painful stimuli (out-of-proportion) Peripheral Pathway Small Caliber (pain/PT) virtually unmyelinated naked endings high threshold AP diffuse projections rapidly sensitize to repetitive stimulation synapse in laminas I-III = warning system = protection via nociception Large Caliber (touch/2PV) myelinated encapsulated endings low threshold AP precise projections adapt to repetitive stimulation synapse in laminas IV-VII = decrease excitability of small caliber fibers = localization Peripheral Nociception Primary Afferent Nociceptors (PANs): pain fibers FAST Aδ-fibers (warning) SLOW C-fibers (protection) Mechanoreceptors and chemoreceptors Chemoreceptors 30% Aδ, 40% C-fibers = “silent nociceptors” become active only with tissue damage or inflammation Peripheral Nociception Noxious stimuli or tissue damage causes release of proteins which irritate and activate PANs PAN Activators: induction of metabolic cascades *Neuropeptides (PANs): substance P, calcitonin gene-related polypeptide (CGRP), and somatostatin Bradykinins (from tissue) Histamines (mast cells) Prostaglandins (cell membrane)-increases pain in the PNS, decreases pain in CNS ATP (damaged muscles): activate receptors on PAN membrane H+ (tissue) Chemokines (immune and nervous system cells) Serotonin (platelets) Peripheral Sensitization The “TRIAD” setup: juxtaposed bare PANs, vessels, and mast cells Tissue Injury: vasodilation → swelling/edema → activation of stretch receptors and immunocyte migration to the region → cytokine release (IL, IF, TNF) Irritation of distal PAN ending → proximal relay to Dorsal Root Ganglia Dorsal Root Reflexes → distal PAN endings release the same type of neuropeptides (substance P and CGRP) that initiated the original nerve irritation “Chemical soup” affecting and activating other adjacent PAN endings → Dorsal Root Reflex Consequently, there is a decreased threshold to activate pain fibers, AND an additional population of pain fibers are “awakened” (silent nociceptor activation) Dorsal Root Reflexes Inflammation -A wave of depolarization enters the dorsal horn and adjacent terminals become depolarized. A greater population of dorsal horn neurons respond to a stimulus at the original site of injury -Small afferent fibers release neuropeptides (SP, CGRP) into the periphery, (antidromically) -Thus, silent nociceptors are activated (40% of C-fibers, 30% of Aδ-fibers) Peripheral Nociception Cascade Schematic borrowed from Frank Willard, PhD Peripheral Sensitization Sequelae Activated PAN stimulate spinal cord pathways via proximal release of: Amino acids: Glutamate and Aspartate into the dorsal horn activates post-synaptic NMDA channels = second messenger system Neuropeptides: Substance P and Calcitonin gene-related peptide into the dorsal horn AND peripheral tissue activates further mast cell degranulation, further vasodilation, & further immunocyte migration in the periphery inflammatory chemical soup develops around adjacent PAN endings activates phosphorylation cascade = second messenger system Peripheral Sensitization Sequelae “The peripheral mechanisms responsible for neuropathic pain are found in the altered gene/protein expression of primary sensory neurons [PANs]. With damage to peripheral sensory fibers, a variety of changes [“plasticity”] in pain-related gene expression take place in dorsal root ganglion neurons.” –H. Ueda (2008) C e r v i c a l Dorsal Root Entry Zone Lateral (pain) & Medial (touch) Lamina I (pain) C o r d Lamina II and III (pain) T o p o g r a p h y Lamina IV (touch) Lamina V and VI (touch) Lamina VII (touch) Dorsal Horn Topography the Laminas: I: prickling, tingling, coolness II/III: burning, itching, nociception “substantial gelatinosa” = lamina II generally does not project axons out of the cord, instead they help integration of multiple segments IV: light touch V/VI: referred pain, peripheral cutaneous, visceral input VII: proprioception from muscles, tendons Implications: in pain states, second messenger systems cause large caliber (touch) fibers to sprout, forming collateral fibers to nociceptive lamina II Spinal Cord Level Pathways Dorsal Horn receives input from: Pain fibers Touch fibers Wide dynamic range (WDR) Receive large receptive fields (A β, A δ, C) and stimuli intensities Modified by descending pathways Selective activation leads to perception of discrete pain (normally) Segmental Control “Gate Control Theory” Large caliber fibers naturally yield collateral fibers, which synapse on inhibitory interneurons and presynaptic PAN terminals. Aδ-PAN terminals in the dorsal horn are surrounded by these GABAnergic synapses. Therefore, gentle touch inhibits our sensation of pain. Brain Schematic borrowed from Frank Willard, PhD Spinal Facilitation Repetitive stimulation of a WDR neuron causes excitotoxicity and cell death. Receptor Field Dynamics Facilitation causes sprouting of PAN terminals to other WDR neurons. When PAN-1 sprouts to WDRN-2, WDRN-2 gains the afferent input from PAN-1’s receptor field in addition to input from PAN-2, thereby doubling its receptor field. Detrimental if the receptor field of PAN-1 is already sensitized May lead to further excitotoxicity of other inhibitory WDR neurons Receptor Field Dynamics PAN 1 WDRN 1 (dying) Receptor Field 1 Sprouting WDRN 2 PAN 2 Receptor Field 2 Spinal Facilitation “Wind-up” Increased Ca+ entry into dorsal horn neuron causes an increase in the cell’s Ca+ permeability, hyperexcitable state Gene induction initiating synthesis of endogenous opioid (Dynorphin) sprouting Ephaptic electrical cross talk spreads excitation to neighboring neurons Activity-dependent plasticity and structural re-organization: Large caliber (touch) fibers sprout, forming collaterals to nociceptive lamina II Altered activity of cord segment Ventral Root Effects: Changes in skeletal muscle tone, ROM, joint positioning Enhanced sympathetic tone Spinal Facilitation: Summary Activity Dependent Plasticity Calcium influx (mediated by NMDA receptors)→ decreased AP threshold, increased excitability, increased sensitivity Excitotoxicity → death of inhibitory interneurons Sprouting from PAN terminals to other WDR neurons → increased receptor field → excitotoxicity → death of other inhibitory interneurons Large fiber collaterals to nociceptive laminas, “wind-up” (due to the second messenger system and gene induction) Dorsal Root Reflexes: antadromic firing of PANs Ascending Pathways Lateral Pain Pathway Localization SC(ALS) to VP thalamus to Primary and Secondary Sensory cortex Medial Pain Pathway carries pain perceived as dull, throbbing, achy, diffuse can be accompanied by nausea, vomiting, fear due to brainstem and limbic connections SC(ALS) to Medial thalamus to Pre-frontal and Anterior Cingulate cortex (Limbic areas) influences two Descending Pain Modulating Pathways Descending Pathways “Endogenous Pain Control” Limbic System influences dorsal horn and interneurons via a Serotonergic pathway. Norepinephrine stimulates the Arousal Center of the Hypothalamus, activating: Sympathetic Nervous System Hypothalamic-Pituitary Axis Cortisol/Glucocorticoids released to facilitate wound healing Oxytocinergic Pathway (?) Selective blockade of Aδ and C-fibers, and glutamate activation The Inflammatory Reflex: -A neuro-physiological mechanism that regulates the immune system -Inflammatory input activates an anti-inflammatory output -Can be under conscious control via higher cortical functions Cholinergic anti-inflammatory mechanisms (activated by limbic system and hypothalamic projections) Acetylcholine inhibits activation of macrophages and cytokine release (TNF-α, IL-1) Ach binds receptors on macrophages and other cytokine producing cells. Binding suppresses pro-inflammatory cytokines. Vagus nerve (90% of fibers are afferents) Stimulated by TNF and other cytokines, mechano- and chemoreceptors, temp sensors, and osmolarity sensors Stimulation prevents inflammation and inhibits cytokine release Summary: Normal Pain Fxn Lateral Pain Pathway: Localization Medial Pain Pathway: Activate Activate inhibitory pathways for pain control arousal system for protection and wound healing Inflammatory reflex acting in peripheral tissues Cholinergic neurons inhibit acute inflammation “Health [is] restored when inflammation is limited by anti-inflammatory responses that are redundant, rapid, reversible, localized, adaptive to changes in input and integrated by the nervous system.” (Tracey 2002) Return to homeostasis Summary: Abnormal Pain Fxn Perpetuation of inflammatory cascade Activation of “silent nociceptors” in the periphery Spinal sensitization: increased input of nociceptive dorsal horn cells Loss of inhibitory interneurons 2° excitotoxicity Increasing receptive field size as previously innocuous sites now evoke response at cord level Wind-up: progressive and sustained partial depolarization of the cell, lowers its threshold, allowing smaller afferent impulses to result in action potentials. Impaired descending pain modulators, ie “inflammatory reflex” Failure to re-establish homeostasis Pain Generators of the Spine Intervertebral Discs HIZ on MRI: 89% specificity for symptomatic annular tear when present at the level of chronic LBP Spinal Stenosis Boney Pathology Compression Fracture Spondylolysis Spondylolisthesis Zygopophysial (Facet) Joints innervated by Medial Branch Nerves Referred Pain Sacral Stress Fx: incidence 2-4% in athletes, up to 20% in runners References Braddom, Randall L (2004) Handbook of Physical Medicine & Rehabilitation. p591-603. Braddom, Randall L (1996) Physical Medicine & Rehabilitation. p876-913. Backonja, Misha-Miroslav (1998) Neurologic Clinics. p755-809. Condes-Lara, M et al (Apr 2006). “Paraventricular hypothalamic influences on spinal nociceptive processing.” Brain Res; 1081(1), p126-137. Duan, B et al. (Oct 2007) “Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity.” Journal of Neuroscience; 27(41), p11139-48. Duric, Vanja and KE McCarson (Oct 2007) “Neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain.” Molecular Pain; 3, p32. Farajidavar, A et al. (2006) “Computational modeling of Abeta fiber wind-up.” Conf Proc IEEE Eng Med Biol Soc; 1, p4975-8. Garcia-Larrea, L and M Magnin (Feb 2008) “[Pathophysiology of neuropathic pain: review of experimental models and proposed mechanisms].” Presse Med; 37(2pt2), p315-340. Gilron, Ian et al. (Aug 2006) “Neuropathic Pain: a practical guide for the clinician.” CMAJ; 173(3), p265-275. Gonzalez, Erwin, chief ed. (2001) Physiologic Basis of Rehabilitation Medicine. P815-845. Hoseini, SS et al (2006). “Sprouting phenomenon, a new model for the role of A-beta fibers in wind up.” Med Hypotheses: 66(4), p805-807. Ivanusic, JJ (16 Feb 2008) “The pattern of Fos expression in the spinal dorsal horn following acute noxious mechanical stimulation of bone.” European Journal of Pain; Epub ahead of print. Levin, ME et al (19 Dec 2007) “Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain.” Pain; Epub ahead of print Lu, VB et al. (Oct 2007) “Neuron type-specific effects of brain-derived neurotrophic factor in rat superficial dorsal horn and their relevance to “central sensitization.’” Journal of Physiology; 584(pt2), p543-563. Mense, S (Dec 2001) ”[Pathophysiology of low back pain and the transition to the chronic state – experimental data and new concepts].” Schmerz; 15(6), p413-417. Merskey H. and N. Bogduk, eds. (1994) Classification of Chronic Pain (2nd ed). Oke, Stacey L. and Kevin J. Tracey (Mar 2008) “From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex.” Journal of Leukocyte Biology; v83, p512-517. References, continued Shah, JP et al. (Jan 2008) “Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points.” Arch Phys Med Rehabil; 89(1), p157-159. Sheets, PL et al (31 Mar 2008) “Differential block of sensory neuronal voltage-gated sodium channels by lacosamide, lidocaine and cabamazepine.” Journal of Pharmacology and Experimental Therapeutics; Epub ahead of date. Schwartz, ES et al (27 Mar 2008) “Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice.” Pain; Epub ahead of print. Sorkin, Linda S. and Mark S. Wallace (Apr 1999) Surgical Clinics of North America. p213-229. Sung, CS and CS Wong (Jun 2007) “Cellular mechanisms of neruoinflammatory pain: the role of interleukin-1beta.” Acta Anaesthesiology Taiwan; 45(2), p103-109. Svensson, Peter et al. (1997) “Cerebral Processing of Acute Skin and Muscle Pain in Humans.” Downloaded from jn.physiology.org on April 8, 2008; p450-460. Takebayashi, T et al (Apr 2006) “Sympathetic afferent units from lumbar intervertebral discs.” The Journal of Bone and Joint Surgery; 88(4), p 554-7. Tracey, Kevin J (Dec 2002) “The inflammatory reflex.” Nature; v420, p853-859. Ueda, H (Apr 2008) “Peripheral mechanisms of neuropathic pain-involvement of lysophosphatidic acid receptor-mediated demyelination.” Molecular Pain; 4(1), p11. Villetti, Gino et al. (2003) “Antinociceptive Activity of the NMDA Receptor Antagonist N-(2-Indanyl)glycinamide Haydrochloride (CHF3381) in Experimental Models of Inflammatory and Neuropathic Pain.” The Journal of Pharmacology and Experimental Therapeutics; 306(2), p804-814. White FA et al (18 Dec 2007) “Chemokines and the pathophysiology of neuropathic pain.” Proc National Academy Sci USA; 104(51), p20151-8 Epub. Willard, Frank H (2001/2006) “Central Pain.” p1-7. Willard, Frank H (2002) “The Nervous System: Acute Pain.” p1-15. Willard, Frank H (2007) “Acupuncture in Women’s Health and Pelvic Pain.” p1-19. Willis, William D Jr. (Oct 2007) “The Somatosensory system, with emphasis on structures important for pain.” Brain Res Review; 55(2), p297-313. Epub 2007 Jun 12. Thank you.