ENERGY LEVELS FOR HOMONUCLEAR DIATOMIC MOLECULES
ENERGY LEVELS FOR HOMONUCLEAR DIATOMIC MOLECULES
What do I have to know?
You will be responsible for being able to write or identify ground and excited state configurations for homonuclear diatomic molecules and their ions and be able to:
I.
Determine whether the molecule is paramagnetic or diamagnetic
II.
Calculate the bond order
III.
From the bond order determine their relative bond length and bond strength
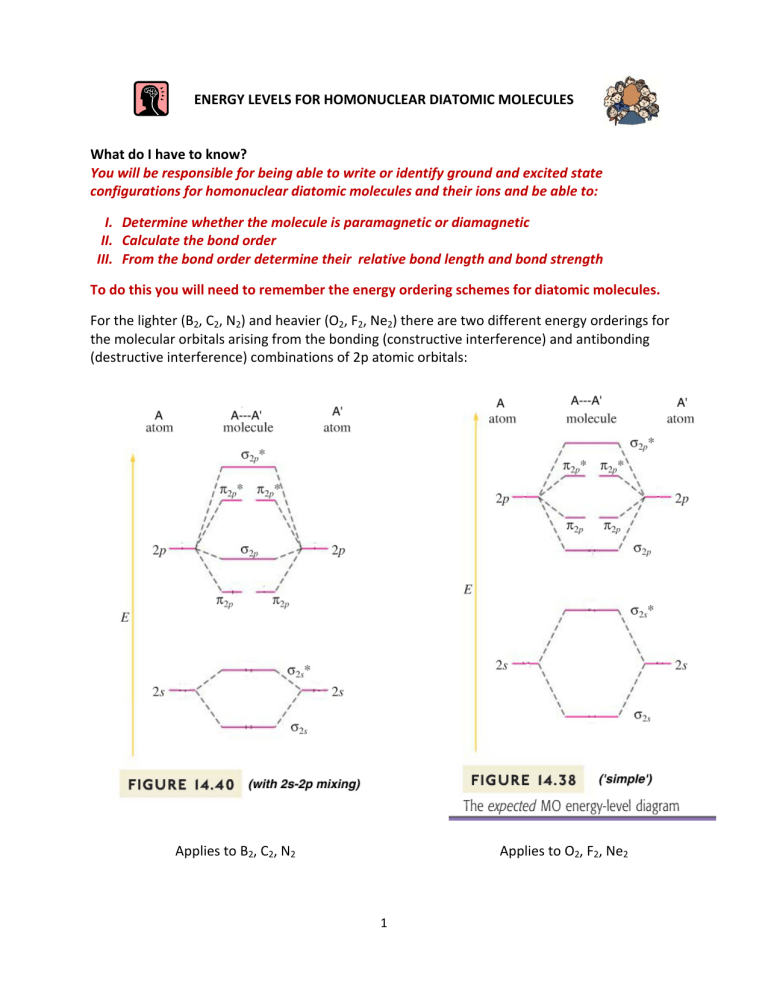
To do this you will need to remember the energy ordering schemes for diatomic molecules.
For the lighter (B
2
, C
2
, N
2
) and heavier (O
2
, F
2
, Ne
2
) there are two different energy orderings for the molecular orbitals arising from the bonding (constructive interference) and antibonding
(destructive interference) combinations of 2p atomic orbitals:
Applies to B
2
, C
2
, N
2
Applies to O
2
, F
2
, Ne
2
1
Remember the two principles that determine whether a.o.’s on two atoms will interact to form bonding and antibonding m.o.’s:
A.
The a.o.’s must have similar energies
B.
The two a.o.’s must ‘overlap’ and interact to have net constructive and destructive interference.
The degree of stabilization by constructive interference (and destabilization by destructive) is determined by the extent of this interaction.
We can ask the following relevant questions:
1.
Why are there a pair of degenerate levels for each of the p
2p
and p
2p
* m.o.’s?
the two side ‐ on interactions (2p y
)
A
ï (2p y
)
A’ and
(2p z
)
A
ï (2p z
)
A’
are equivalent with the only difference being their directions in space; constructive interference leads to two m.o.’s of equal (lower) energy and destructive interference leads to two p
2p
* of equal p
2p
(higher) energy.
2.
Why in the “expected” is the energy of the s
2p
is lower than that of the p
2p
in the ‘simple’
energy level scheme (Fig 14.38)?
the
(2p y
end
)
A
‐ on
ñ (2p
interactions y
)
A’ or
(2p z
)
A
of the (2p x
)
A
ñ (2p x
)
A’
ñ (2p z
)
A’
(principle B).
are stronger than the side ‐ on interactions of
3.
What is meaning of 2s ‐ 2p mixing and why is it more important for B
2
, C
2
, N
2 than for
O
2
, F
2
, Ne
2
?
A 2s orbital on atom A will have net interference (‘overlap’) with a 2p x
on atom A’.
yes for 2s ‐ 2p x
we can have net
constructive or destructive
interference
2s on A 2px on A’
and thus they could contribute to the same m.o.
(principle B) if they had similar energies
(principle A) .
In O
2
, F
2
, Ne
2
the larger Z (nuclear charge) makes the energy of the 2s atomic orbital much lower than the 2p atomic orbital and thus the 2s and 2p on A and A’ DO NOT mix when forming m.o.’s (simple scheme no 2s ‐ 2p mixing) .
However in B
2
, C
2
, N
2
the energies of the 2s and 2p atomic orbitals are much closer and thus the s
2s
and s
2s
* m.o.’s contain some contribution from 2p x
a.o.s
and the s
2p
and s
2p
* m.o.’s contain some contribution from 2s a.o.’s.; i.e.
THERE IS 2s ‐ 2p mixing for B
2
, C
2
, N
2
2
4.
What is the effect of 2s ‐ 2p mixing on the energy level diagram?
The 2p x
provides bonding (constructive) interactions in the s
2s
and s
2s
* m.o.’s
LOWERING their energies.
The 2s participates in the s
The energies of the
2p
and s
2p
* m.o.’s with antibonding (destructive) interactions and thus RAISES the energies of the s
2p
and s
2p
* m.o.’s.
p
2p
and p
2p
* m.o.’s are unaffected since there is no 2s mixing with the 2p y
or 2p z
.
The
result
TO
REMEMBER
is
that
for
B
2
,
C
2
,
N
2
than
the
s
2p.
the
p
2p
has
a
lower
energy
5.
How can one experimentally verify that the 2s ‐ 2p scheme mixing applies to B
2
and C
2
?
One can ask “what would be the predictions for the ground state electronic configurations of B
2
and C
2 in the two energy level schemes”?
B
2
: simple: ( s
1s
)
2
( s
1s
*)
2
( s
2s
)
2
( s
2s
*)
2
( s
2p
)
2
î DIAMAGNETIC
2s ‐ 2p mixing: ( s
1s
) 2 ( s
1s
*) 2 ( s
2s
) 2 ( s
2s
*) 2 ( p
2p
) 2
≠
≠ î PARAMAGNETIC
and B
2
is observed to be PARAMAGNETIC !!
C
2
: simple: ( s
1s
)
2
( s
1s
*)
2
( s
2s
)
2
( s
2s
*)
2
( s
2p
)
2
( p
2p
2s ‐ 2p mixing: ( s
1s
)
2
( s
1s
*)
2
( s
2s
)
2
( s
2s
*)
2
( p
2p
)
4 ≠Ø
)
2
≠
≠
î
PARAMAGNETIC
≠Ø î DIAMAGNETIC
and C
2
is observed to be DIAMAGNETIC !!
6.
How can one experimentally verify that the simple scheme applies to O
2
and F
2
?
The configurations for these molecules are the same for the two schemes (except for reversal of the inner s
2p
and p
2p
levels) and thus would not predict differing numbers of unpaired electrons.
Experimental verification requires analysis of the electronic excitation spectra of these molecules.
THE ENERGY LEVELS DO CORRESPND TO THE SIMPLE SCHEME!!
3