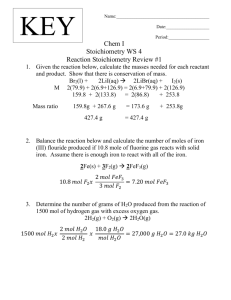
Worksheet % Composition Empirical formulas
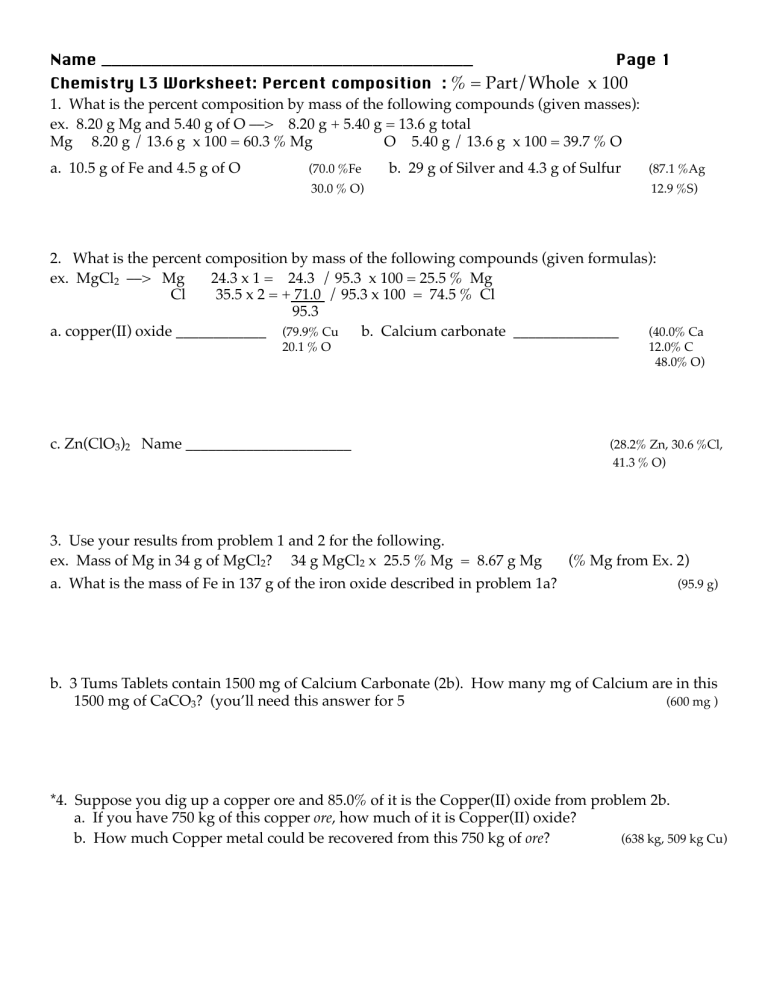
Name _____________________________________ Page 1
Chemistry L3 Worksheet: Percent composition : % = Part/Whole x 100
1. What is the percent composition by mass of the following compounds (given masses): ex. 8.20 g Mg and 5.40 g of O ––> 8.20 g + 5.40 g = 13.6 g total
Mg 8.20 g / 13.6 g x 100 = 60.3 % Mg !
O !
5.40 g / 13.6 g x 100 = 39.7 % O
!
a. 10.5 g of Fe and 4.5 g of O !
(70.0 %Fe !
b. 29 g of Silver and 4.3 g of Sulfur !
(87.1 %Ag
30.0 % O) !
12.9 %S)
2. What is the percent composition by mass of the following compounds (given formulas):
!
!
ex. MgCl
2
––> Mg 24.3 x 1 = 24.3 / 95.3 x 100 = 25.5 % Mg
Cl 35.5 x 2 = + 71.0 / 95.3 x 100 = 74.5 % Cl
95.3
!
!
a. copper(II) oxide ____________ !
(79.9% Cu !
b. Calcium carbonate ______________ !
(40.0% Ca
20.1 % O !
12.0% C
48.0% O)
!
c. Zn(ClO
3
)
2
Name ______________________ !
(28.2% Zn, 30.6 %Cl,
41.3 % O)
3. Use your results from problem 1 and 2 for the following.
ex. Mass of Mg in 34 g of MgCl
2
? 34 g MgCl
2
x 25.5 % Mg = 8.67 g Mg (% Mg from Ex. 2) a. What is the mass of Fe in 137 g of the iron oxide described in problem 1a?
!
(95.9 g) b. 3 Tums Tablets contain 1500 mg of Calcium Carbonate (2b). How many mg of Calcium are in this
1500 mg of CaCO
3
? (you’ll need this answer for 5 !
(600 mg )
*4. Suppose you dig up a copper ore and 85.0% of it is the Copper(II) oxide from problem 2b.
!
a. If you have 750 kg of this copper ore , how much of it is Copper(II) oxide?
!
b. How much Copper metal could be recovered from this 750 kg of ore ? !
(638 kg, 509 kg Cu)
*5. The RDI (Recommended Daily Intake) for Calcium for women is 1000 mg of Calcium Page 2 per day. Tums contains Calcium Carbonate (#2b, 3b). "CitriCal" Calcium supplement is Calcium
Citrate . Let’s find out which gives us more Calcium: 3 Tums tablets (3b) or 3 Citrical Tablets.
a. The Citrate ion is C
6
H
5
O
7
–3 . Write the formula for Calcium Citrate _____________________.
b. Determine the % Calcium in Calcium Citrate.
c. Assume a 750 mg “CitriCal” tablet is 60.0% Calcium Citrate (the rest is starch, sugar, etc.). How many mg of Calcium do you get from 3 of these tablets? !
(a. Ca
3
(C
6
H
5
O
7
)
2; b. 24.1 % Ca; c. 325 mg Ca) d. So which gives you more Calcium, 3 Tums (3b) ______mg Ca or 3 Citrical Tablets? ______mg Ca
Empirical Formulas: Lowest whole number ratio
6. Determine the Empirical Formula of the compounds with the following % compositions. example: 52.2 % C, 13.0 % H, 34.8 % O
!
52.2 g C x 1 mol = 4.35 mol C !
13.0 g H x 1 mol = 13.0 mol H !
34.8 g O x 1 mol = 2.18 mol O
12 g C !
1 g H !
16 g O divide by smallest (2.18) 2 mol C !
6 mol H !
1 mol O so Empirical Formula = C
2
H
6
O
6. Determine the Empirical Formula of the compounds with the following % compositions. a. 85.6 % C, 14.4 % H !
(CH
2
) !
b. 62.1 % C, 10.3 % H, 27.6 % O !
(C
3
H
6
O)
Check list of special fractions for c and d c. 81.8 % C, 18.2 % H !
(C
3
H
8
) !
d. 47.4% C, 10.6 % H, 42.0% O !
(C
3
H
8
O
2
)
7. 20.00 g of a compound contains 17.8 g Cu and 2.2 g O . Determine the empirical formula and name the compound. (need a Roman numeral ) ______________________________ !
(Cu
2
O)
8. 10.0 g of an ionic compound contains 4.48 g K, 1.84 g S, and 3.68 g O. What is the Empirical formula and the name of the compound? (hint: check list of polyatomic ions) !
(K
2
SO
4
)
Chem L3 Empirical formulas cont. : Name ________________________ Page 3
1. These Ionic Empirical formulas look pretty strange. To figure out their names : Add parentheses and check your list to figure out the polyatomic ions . Then determine the charge (Roman numeral) for the Metal . Then you can name them. Ex: SnN
2
O
4
becomes Sn(NO
2
)
2
, so name is Tin(II) nitrite
!
a. CoPO
4
_________________________________ !
c. NiC
3
N
3
___________________________________
!
b. MnN
2
O
6
________________________________ !
d. Fe
2
S
3
O
9
__________________________________
Molecular Formulas: Example: Empirical formula CH
2
, experimental formula weight = 56 g/mol
!
!
CH
2
= 14 ––> _56 g_ = 4 so the molecular formula is (CH
2
)
4
or C
4
H
8
14 g
2. Determine the molecular formula of the following molecular compounds.
a. empirical formula C
2
H
5
, experimental formula weight = 58.0 g/mol !
(C
4
H
10
) b. empirical formula C
2
H
3
O, experimental formula weight = 172 g/mol !
(C
8
H
12
O
4
)
3. You are called in to investigate a murder. You analyze the substance found at the site and find its percent composition is 40.0% C, 6.6% H, 53.4% O. You then find its experimental formula weight =
180 g/mol. What is the molecular formula for this compound?
!
(C
6
H
12
O
6
)
(1a. Cobalt(III) Phosphate; b. Manganese(II) nitrate; c. Nickel(III) Cyanide; d. Iron(III) sulfite)
Density, Formula weight : !
Formula weight = Density x 22.4 L !
or _g_ = g x 22.4 L of gases at STP !
of a gas !
of Gas !
mol !
mol !
L !
mol
4. What is the density ( in g/L) of these gases at STP? (divide FW by 22.4 L) The density of air is
1.19
g/L. Would each gas "float" in the air or "sink"? a. CH
4
!
(0.714 g/L) !
b. SO
3
!
(3.57 g/L)
5. 1.25 L of a gas (at STP) has a mass of 2.46 g. a. Calculate the Density of the gas in g/L.
!
b. Calculate the formula weight of the gas (in g/mol). Might this gas be SO
3
, NO, or N
2
O ?
!
! !
(1.97 g/L, 44 .1 g/mol)
6a. 500 mL of a gas (at STP) has a mass of 0.625 g. Calculate the density of the gas in g/L. b. Calculate the formula weight of the gas. Could this gas be carbon monoxide or carbon dioxide?
!
! !
(1.25 g/L, 28.0 g/mol)
Lab Application Review problem: !
7. The mass of an empty crucible is 13.400 g. You add 0.600 g of Iron metal. You heat the crucible and the Iron reacts with Oxygen in the air. The mass of the product + crucible is 14.257 g. !
(answers below) a. How many moles of Iron did you start with?
b. What is the mass of product in the experiment?
Empty
Crucible
Iron
Crucible + product
Product
Oxygen in product
Mass, grams
Page 4
#SF Moles #SF
XXXXX XX
XXXXX XX
XXXXX XX c. What is the mass of oxygen in the product? Then Change the mass of Oxygen to moles of oxygen.
d. Determine the empirical formula for the product. Then Name the product (Roman numeral!) e. Calculate the experimental % Iron by mass in the product. f. How many grams of Iron would be in 42.7 g of this Iron Oxide?
(Ans for #4a. 0.0107 mol, b. 0.857 g, c. 0.257 g , 0.0160 mol, d. Fe
2
O
3
, Iron(III) oxide, e. 70.0 % Fe, f. 29.9 g Fe)
Review problems: Moles, % Composition, empirical formulas
8. a. You analyze a gas and find it is 82.8 % carbon and 17.2 % hydrogen. What is its empirical formula?
!
(C
2
H
5
)
!
b. At STP, 1.29 g of this gas occupies a volume of 0.500 L. What are the density and experimental formula weight of the gas? !
(2.59 g/L, 57.8 g/mol)
!
c. What is the molecular formula for the gas? !
(C
4
H
10
)
!
9. What is the % composition by mass of Cobalt(II) Nitrate? Formula = ________________________
!
(32.2% Co, 15.3 %N, 52.5% O)
More Review problems: % Composition, empirical formulas
1. a. What is the percent composition by mass of NaHCO
3
?
!
(27.4 % Na
Page 5
, 1.1 %H, 14.3 %C, 57.1 %O)
!
!
!
b. An Alka seltzer tablet is about 38.0 % NaHCO
3
. If a tablet has a mass of 5000 mg, how many mg of sodium do you get from "drinking" 2 of these antacid tablets? !
(1041 mg)
!
c. How many milligrams of Sodium are in 400 mg of milk of magnesia antacid, Mg(OH)
2
?
!
d. It is recommended that we keep our sodium intake below about 1500 mg a day. Which one is better for people on "low sodium" diets: Milk of magnesia or Alka seltzer? (ans below)
2. At a local drug bust, you find 30.00 g of some green crystals. You analyze this substance and find out that 8.70 g is Nickel, 7.11 g is Sulfur and 14.19 g is Oxygen.
!
a. What is the percent composition by mass of this compound?
!
(29.0 %Ni, 23.7 %S, 47.3%O)
!
b. What is the empirical formula and name of this compound? !
(Ni
2
S
3
O
12
, name below)
3. A gas has an empirical formula of CH
2
. At STP, 150 mL of the gas has a mass of 0.281 g.
!
a. Calculate the density and formula weight of the gas. b. Determine the molecular formula for the gas.
!
(1.87 g/L, 42.0 g/mol, C
3
H
6
)
4. You heat up 0.500 g of Manganese and it reacts with oxygen. The mass of the product is 0.791
g.
What is the formula and name (Roman numeral) of the product?
!
(MnO
2
, name below)
(1. c & d. there’s no Na in Mg(OH)
2
:) ; 2. Nickel(III) sulfate; 4. Manganese (IV) oxide) )
Extra Review problems: Moles, % Composition, empirical formulas p 6
1. You analyze a compound and find this per cent composition: 55.8% C, 7.0% H, 37.2% O. Then you find its actual formula weight is 172 g/mol. What are the empirical and molecular formulas of the compound? !
(C
2
H
3
O , C
8
H
12
O
4
)
2. You have some orange crystals which are 26.5 % K, 35.4 % Cr and 38.1 % O. What is the empirical formula and name of this compound? !
(K
2
Cr
2
O
7
)
3. 800 mL of a gas at STP has a mass of 2.54 g. What is its formula weight? If this gas is a diatomic element, which element is it?
!
(71 g/mol)
4. You analyze some Copper ore for your mining company. 3.98 g of a copper sulfide contains 3.18 g of Copper. a. What is the % composition by mass of this copper sulfide? b. What is its empirical formula and name? (Roman numeral for the copper) !
(79.9% Cu, 20.1 % S, Cu
2
S, copper (I) sulfide)
!
b. What mass of Copper can be obtained from 150 g of this Copper sulfide? !
(120 kg)
!
c. You have some Copper Ore that contains 37.5 % this Copper sulfide. How many grams of
Copper are in 65.3 kg of this Copper Ore?
!
(19.6 kg)
5. The empirical formula of a compound is C
2
HCl and its actual formula weight is 181.5 g/mol. What is its molecular formula?
!
(C
6
H
3
Cl
3
)