Document
advertisement

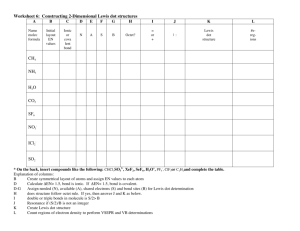
Name _________________________ Lewis Dot structures of Ionic Compounds Ionic compounds generally contain a metal and a nonmetal in the formula. The metal cations come first in the formula, while the nonmetal containing anions are second in the formula. The only exception to this is when the cation is the ammonium ion (NH4+), which does not contain a metal. There are two basic types of ionic compounds: (1) Binary compounds, or compounds with just two types of atoms in the formula, i.e. NaCl; and (2) Terinary compounds, or compounds that have three or more types of atoms in the formula. Generally the terinary compounds result from the combination of a monoatomic cation (or ammonium ion) and a polyatomic anion, i.e. Na2CO3. To write the Lewis dot structure of a binary ionic compound simply write the Lewis dot structure(s) of the cation(s), including brackets and the charge, followed by writing the Lewis dot structure(s) of the anion(s), including brackets and charges. For example, the Lewis dot structure of NaCl is shown in Figure 1a. In order to draw the Lewis dot structures of polyatomic ions, simply follow the rule established for molecular compounds, being sure to add or subtract electrons resulting from the charge of the ion, as shown in Figure 1b for NH4Cl. Be sure to draw the Lewis structure for each ion in the formula. For example in Li2O, there are two lithium ions and only one oxide ion. .. [Na]+[:Cl:] .. H .. H-N-H + [:Cl:] .. H (a) (b) Figure 1. (a) NaCl and (b) Na2CO3. Draw Lewis dot structures for the following ions. 1. F- 2. Na+ 3. O2- 4. N3- 5. NH4+ 6. BF4- 7. OH- 8. S2- 9. O22- 10. Mg2+ © Van Der Sluys, 2004 Name _________________________ Lewis Dot structures of Ionic Compounds Identify the cation and anion in each of the following ionic compounds and draw the Lewis dot structure for each compound. 11. Na2O 12. NH4F 13. NaOH 14. MgF2 15. Na2O2 16. LiF 17. NH4OH 18. CaCl2 19. Al(OH)3 20. CaO © Van Der Sluys, 2004 Name _________________________ Answers .. 1. [:F:] .. 2. [Na]+ .. 23. [:O:] .. .. 34. [:N:] .. H 5. [H-N-H]+ H .. + .. :F: .. :F-B-F: .. .. :F: .. 6. .. 7. [:O-H] .. .. 8. [:S:] .. 2.. .. 9. [:O-O:] .. .. 210. [Mg]2+ .. 211. [Na]+ [Na]+ [:O:] .. H .. 12. [H-N-H]+ [:Cl:] .. H .. + 13. [Na] [:O-H] .. .. .. 14. [Mg]2+ [:F:] .. - [:F:] .. .. .. 215. [Na]+ [Na]+ [:O-O:] .. .. .. 16. [Li]+ [:F:] .. H .. 17. [H-N-H]+ [:O-H] .. H .. .. 18. [Ca]2+ [:Cl:] [:Cl:] .. .. .. .. .. 19. [Al]3+ [:O-H] [:O-H] [:O-H] .. .. .. .. 220. [Ca]2+ [:O:] .. .. © Van Der Sluys, 2004 Lewis Dot structures of Ionic Compounds