7-3 Nuclear Reactions
advertisement

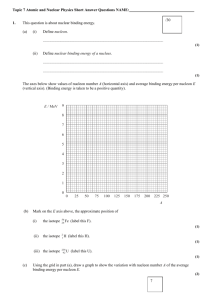
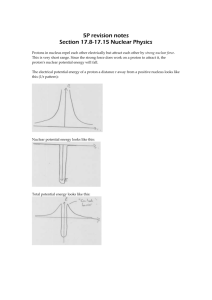
Nuclear Reactions Apart from naturally occurring radioactive decay, it is also possible to produce a nuclear reaction by firing a particle into a nucleus. This produces an unstable intermediate which then breaks down. Particles which are commonly used are neutrons, protons and alpha particles. The Rules * Conservation of nucleon number (total of mass numbers does not change) * Conservation of charge (total of atomic numbers does not change) * Conservation of Lepton Number (e+ plus or e- plus ) * Conservation of mass-energy Examples: An alpha particle strikes a nitrogen-14 nucleus A neutron strikes a lithium-6 nucleus The Unified Mass Unit (u) The kilogram is inconveniently large for work with individual particles, so the unified mass unit is used. Definition: 1u is 1/12 of the mass of an atom of carbon-12 (1u=1.66 x 10-27 kg.) Mass of proton = 1.007 276 u By definition, 1 mole is the Mass of neutron = 1.008 665 u number of atoms in 12g of Mass of electron = 0.000 549 u carbon-12. This is Avogadro's Mass of alpha = 4.001 506 u number: 6.023 x 1023 of Mass of deuteron = 2.013 553 u atoms. Energy Units The joule is also very large for measuring the energy of individual particles. In this case, the electron-volt is used. This is the energy difference when 1 electron charge (rather than 1 coulomb) is moved across a potential difference of 1 volt. Since 1C = 6.25 x 1018 e, 1J = 6.25 x 1018 eV or 1eV = 1.6 x 10-19 J Actually, the eV is a bit too small, so keV or MeV are quite common. 1 Mass-Energy Equivalence Einstein investigated the relationship between mass and energy and concluded that they are different aspects of the same thing. One interpretation of this relationship is that "energy has mass". Einstein's Mass-Energy Equation E = mc2 1u of mass is equivalent to 931.5 MeV of energy. Sometimes the unit MeV/c2 is used for mass. This is the amount of mass 1 that has an energy equivalent of 1 MeV. i.e. /931.5 u 2 (GeV/c is also used.) This has an important effect when particles travel close to the speed of light. If the kinetic energy is large, its own mass is added to the "rest mass" of the particle. The figures given above are "rest masses." Mass Defect and Nuclear Binding Energy The mass of a nucleus is less that the total mass of the individual particles that it is made from. This difference is called the "mass defect." The particles in the nucleus are held together by the strong nuclear interaction. To break them apart would require considerable work to be done against this attractive force. This energy difference has a noticeable amount of mass, given by Einstein's equation. The nucleus has a lower potential energy level than the separate components. This energy difference, that corresponds to the mass defect is called the "nuclear binding energy." e.g. Mass of 2p + 2n = 2 x 1.007 276 + 2 x 1.008 665 = 4.031 882 u Mass of a He nucleus (alpha particle) = 4.001 506 u Mass defect = 4.031 882 - 4.001 506 = 0.030 376 u Binding energy = 0.030 376 x 931.5 = 28.30 MeV or 4.5x10 -12 J Often the binding energy per nucleon is required. In this case a helium nucleus has 4 nucleons, so 28.30 ÷ 4 = 7.08 MeV per nucleon. 2 Binding Energy per Nucleon When considering the theoretical assembly of small nuclei, it is easy to see that the attractive force of the strong nuclear interaction is the source of the nuclear binding energy. However, when very large nuclei are considered, it becomes more important to look at the binding energy per nucleon. Adding an extra nucleon may increase the total amount of binding energy, but the average per nucleon decreases, making the nucleus less stable rather than more stable. In fact, the most stable nucleus is iron. This means that a process of joining small nuclei together (fusion) will release energy. Also, splitting large nuclei (fission) will release energy. Nuclei larger than iron can only have been created in the first place with a huge energy input (from supernova explosions.) Fusion Fusion of small nuclei is the source of the energy given out by stars. It is the process by which all the elements from Lithium to Iron were created, as only hydrogen and helium were formed directly in the big bang. While there are different mechanisms, the overall hydrogen fusion reaction in a star like the sun is: Mass on left side = 4 x 1.007 276 = 4.129 104 Mass on right side = 4.001 506 + 2 x 0.000 548 = 4.002 602 Decrease in mass = 4.129 104 - 4.002 602 = 0.126 502 Energy released = 0.126 502 x 931.5 = 117.8 MeV The same principle is used in the thermonuclear (hydrogen bomb) but has not yet successfully been used for power generation. The problem is that the particles must be given a great deal of energy to begin reacting. The positively charged particles must collide in spite of their electrostatic repulsion. Once they react, much more energy is released and the reaction can continue explosively. A controlled reaction, or preferably a "cold fusion" mechanism is needed to develop an industrial application. 3 Fission Uranium-235 decays by alpha emission. However,another possibility is that a U-235 nucleus may spontaneously break into two smaller parts, releasing several neutrons in the fission reaction. If a neutron strikes a U-235 nucleus and is absorbed, U236 is formed momentarily, but breaks down immediately into smaller parts, once again releasing several neutrons. If one or more of these neutrons should effectively strike another nucleus, the reaction rate will quickly increase - leading to a chain reaction. Slow neutrons are most effective in initiating further fission reactions. The principle of the atomic bomb is simply to bring several smaller "subcritical" masses of U-235 or another suitable nuclide (fissile material) together into a "critical mass" and hold it together long enough for a chain reaction to build up. Conventional explosives can be used to achieve this. In a nuclear reactor, a moderating material, often graphite or water, is used to slow the neutrons and make them more effective, and control rods are inserted into the "pile" to control the reaction rate by absorbing excess neutrons. In this way the reaction rate can be balanced with the rate at which energy is removed by the heat-exchange material. 4