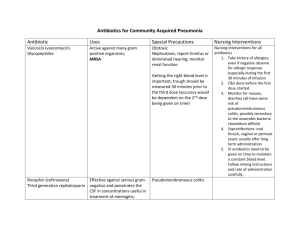
Antimicrobial chemotherapy
advertisement

Antimicrobial Chemotherapy Part I Antimicrobial Chemotherapy • • • • • Use of drugs to combat infectious agents Antibacterial Antiviral Antifungal Antiparasitic Antimicrobial Chemotherapy • Differential(selective) toxicity: based on the concept that the drug is more toxic to the infecting organism than to the host • Majority of antibiotics are based on naturally occurring compounds • or may be semi-synthetic or synthetic What is the ideal antibiotic • Have the appropriate spectrum of activity for the clinical setting. • Have no toxicity to the host, be well tolerated. • Low propensity for development of resistance. • Not induce hypersensitivies in the host. What is the ideal antibiotic • Have rapid and extensive tissue distribution • Have a relatively long half-life. • Be free of interactions with other drugs. • Be convenient for administration. • Be relatively inexpensive Principles / Definitions • Spectrum of Activity: Narrow spectrum - drug is effective against a limited number of species Broad spectrum - drug is effective against a wide variety of species • Gram negative agent Gram positive agent Anti-anaerobic activity Principles / Definitions • Minimum Inhibitory Concentration (MIC) - minimum concentration of antibiotic required to inhibit the growth of the test organism. • Minimum Bactericidal Concentration (MBC) - minimum concentration of antibiotic required to kill the test organism. • • • • Bacteriostatic Bactericidal Time dependent killing Concentration dependent killing Principles / Definitions • Treatment & prophylaxis • Prophylaxis - antimicrobial agents are administered to prevent infection • Treatment - antimicrobial agents are administered to cure existing or suspected infection Combination Therapy • To prevent the emergence of resistance - M.tuberculosis • To treat polymicrobial infections • Initial empiric therapy • Synergy Combination Therapy • Why not use 2 antibiotics all the time? • • • • Antagonism Cost Increased risk of side effects May actually enhance development of resistance inducible resistance • Interactions between drugs of different classes • Often unnecessary for maximal efficacy What influences the choice of antibiotic? • • • • Activity of agent against proven or suspected organism Site of infection Mode of administration Metabolism and excretion – renal and hepatic function • Duration of treatment / frequency of dose • Toxicity / cost • Local rates of resistance How do antimicrobial agents work • must bind or interfere with an essential target • may inhibit or interfere with essential metabolic process • may cause irreparable damage to cell Targets of antibacterial agents • Inhibit cell wall production - penicillin binding proteins • Inhibit protein synthesis - bind 30s or 50s ribosomal subunits • Inhibit nucleic acid synthesis - binding topoisomerases / RNA polymerase • Block biosynthetic pathways - interfere with folate metabolism • Disrupt bacterial membranes - polymixins Antimicrobial resistance • Resistance: the inability to kill or inhibit the organism with clinically achievable drug concentrations • Resistance may be innate (naturally resistant) • Resistance may be acquired - mutation - acquisition of foreign DNA Antimicrobial resistance • Factors which may accelerate the development of resistance - inadequate levels of antibiotics at the site of infection - duration of treatment too short - overwhelming numbers of organisms - overuse / misuse of antibiotics Antimicrobial resistance General mechanisms of resistance Altered permeability Inactivation / destruction of antibiotic Altered binding site Novel (new) binding sites Efflux (pumps) mechanisms Bypass of metabolic pathways Antimicrobial Chemotherapy part II Synthesis of peptidoglycan Action of penicillins and cephalosporines Action of vancomycin from Assembling the Peptide Cross-Links in Peptidoglycan Action of Tetracyclins Action of macrolids Action of Aminoglycosides Action of Aminoglycosides Amphotercin B - colistin Imidazolen - Nystatin polymyxins sulfonamides sulfonamides Action of bacterial enzyme • Without the antibiotic binding to a bacterial enzyme, the activate site of the enzyme is able to react with its substrate. Action of antibiotics on enzyme • When an antibiotic binds to a bacterial enzyme, it may alter the activate site of the enzyme and prevent it from reacting with its substrate. Action of oxazolidones • The oxazolidinones (linezolid) bind to the 50S ribosomal subunit and interfere with its binding to the initiation complex. Initiation Complex Bacterial resistance • Genetic mutations • Acquiring resistance from other bacteria through: conjugation Transformation Transduction trosposons • • • • • • 1. producing enzymes: Penicillinase Acetylase Adenylase Phosphorylase Chloramphenicol acetyl transferase • 2. changes in permeability: • Polymyxines • Tetracyclins 3.Change in receptors of drug • PBPs • 30 S subunite of ribosome • 50 S subunite of rifosome • 4. change in metabolisme • use of folic acid instead of PABA Antibiotic Classes • Cell Wall Active Agents bactericidal, time dependent killing • B-lactams - penicillins / cephalosporins / - cephamycins / carbapenems • Glycopeptides - vancomycin / teicoplanin - gram positive agents Structure of -lactam drugs Penicillins • Penicillin G / V - good gram positive (not Staph) -moderate anaerobic activity • Synthetic penicillins (Ampicillin) - good gram positive (not Staph) - moderate gram negative (not Pseudomonas) • Anti-staphylococcal penicillins - Cloxacillin • Anti-pseudomonal penicillins - Piperacillin Cell Wall Active Agents • B-lactams bind to “penicillin binding proteins” (PBP) -PBP are essential enzymes involved in cell wall synthesis -weakened / distorted cell wall leading to cell lysis and death • Glycopeptides bind to the terminal D-ala of nascent cell wall peptides and prevents cross-linking of these peptide to form mature peptidoglycan Vancomycin: Mechanism of Action • Inhibit peptidoglycan synthesis in bacterial cell wall by complexing with the D-alanyl-D-alanyl portion of the cell wall precurser 2L-ala racemase 2D-ala ligase (ddl) D-ala-D-ala UDP-L-ala-D-glu-L-lys adding enzyme carboxypeptidase -L-ala-D-glu-L-lys-D-ala-D-ala transpeptidase -L-ala-D-glu-L-lys-D-ala-D-ala -L-ala-D-glu-L-lys-D-ala-D-ala transglycosidase -L-ala-D-glu-L-lys-D-ala-D-ala UDP-L-ala-D-glu-L-lys-D-ala-D-ala pentapeptide-- --L-ala-D-glu-L-lys--D-ala-D-ala vancomycin Cell Wall Active Agents • B-lactam resistance 1. Production of a B-lactamase (most common) 2. Altered PBP (S.pneumoniae) 3. Novel PBP (MRSA) 4. Altered permeability • Glycopeptide resistance - primary concern is Enterococcus / S.aureus - altered target - bacteria substitutes D-lac for D-ala - vancomycin can no longer bind Cephalosporins • History – Discovered in sewage in Sardinia in the mid 1940s. – Cephalosporium sp was recovered and proved a source of cephalosporin. – Subsequently, four generations of cephalosporins have emerged. Cephalosporins • 1st generation- mainly gram pos, some gram neg (cefazolin) 2nd generation- weaker gram pos, better gram neg (cefuroxime) 3rd generation - excellent gram neg, some gram pos (ceftriaxone) 4th generation - excellent gram neg, good gram pos (cefepime) First-Generation Cephalosporins: What do they cover? • Cefazolin (Kefzol) and cephalexin (Keflex) – Activity includes: • Methicillin susceptible staphylococci • Streptococci excluding enterococci • E. coli, Klebsiella sp., and P. mirabilis • Many anaerobes excluding B. fragilis Where do you think they should be used? • • • • Simple mixed aerobic infections. In penicillin allergic (not immediate) patients. Surgical prophylaxis. Convenience drug for S. aureus and streptococci? What about second generation cephalosporins? • Cefuroxime • Cefoxitin/cefotetan – Think Haemophilus in – 1st generation plusaddition to 1st anaerobes generation specturm – A mixed, non-serious – A respiratory drug infection surgeon drug – Think cefazolin/metro which is what we would use Third-Generation Cephalosporins • Cefotaxime, ceftriaxone (IV) – Enhanced activity against Enterobacteriaceae – Enhanced activity against streptococci, including penicillin resistant S. pneumoniae. – Long half life favors ceftriaxone – Less diarrhea favors cefotaxime • Ceftazidime (IV) – Active against P. aeruginosa. – Decreased activity against gram positive cocci. Fourth generation cephalosporins • Cefepime – Marginal improvements – Not available at the QE II Carbapenems: What don’t they get? • Everything except: – MRSA and MRSE – Enterococcus faecium – Stenotrophomonas maltophilia – Burkholderia cepacia When to use carbapenems? • Life threatening polymicrobial infections – Intra abdominal sepsis in ICU esp nosocomial in origin – Gram negative/ nosocomial pneumonia in intubated patient • Monotherapy of febrile neutropenia (high risk patients) What are the beta-lactamase inhibitors? • Clavulanate (with amoxicillin or ticarcillin) • Tazobactam (with piperacillin) What additional bugs do they cover? • • • • • S. aureus H. influenzae Neisseria sp. Bacteroides fragilis E. coli and Klebsiella • Not better for Pseudomonas or Enterobacter Inhibitors of protein synthesis • Ribosomes are the site of protein synthesis • many classes of antibiotics inhibit protein synthesis by binding to the ribosome • binding may be reversible or irreversible • Macrolides, ketolides, lincosamides, streptogramins Tetracyclines Aminoglycosides Inhibitors of protein synthesis • Macrolides (erythromycin, clarithromycin, azithromycin) - primarily gram positive, mycoplasma, chlamydia - bacteriostatic, time dependent killing Lincosamides (clindamycin) - gram positive, anaerobic activity • Resistance (acquisition of a gene) - M phenotype: macrolides only efflux - MLSB phenotype: macrolides, lincosamides, streptogramins target site modification constitutive, inducible Inhibitors of protein synthesis • Aminoglycosides: gentamicin, tobramycin, amikacin - excellent gram negative, moderate gram positive - bactericidal, concentration dependent • Resistance Primarily due to aminoglycoside modifying enzymes Inhibitors of nucleic acid synthesis • Fluoroquinolones (ciprofloxacin, norfloxacin, levofloxacin, moxifloxacin) - bactericidal, concentration dependent - bind to 2 essential enzymes required for DNA replication - DNA gyrase and topoisomerase IV - gram pos and gram neg - atypical bacteria, some have anaerobic activity • Resistance - altered permeability (porin channels) - altered target site - efflux Inhibitors of metabolic pathways • Trimethoprim/sulfamethoxazole (Septra, TMP/SMX) - good gram negative, some gram positive • block folic acid synthesis at two different points TMP and SMX act synergistically • Resistance may arise if the organism can “bypass” the pathway making it redundant Mechanism of action of TMP-SMX