resting potential 2014

The Structure of the Cell
Membrane
Resting Membrane Potential
Structure of the cell membrane.
Resting membrane potential.
• The Nernst equation.
• Donnan potential.
• The Goldman-Hodgkin-Katz equation
11.11.2014.
Phospho lipids
The main component of the biological membranes.
Phospholipid = diglyceride (glycerine+fatty acid) + phosphate group + organic molecule (e.g. choline).
Polar – head
(hydrophilic)
Non-polar – tail
(hydrophobic)
⇒
„water soluble fat” phosphatidil – choline
14/11/2014
1
Irving Langmuir
American physico-chemist
1932 Nobel-price in chemistry
•
1917 – lipids form a monolayer on the surface of the water polar heads (hydrophilic) – oriented
toward the water nonpolar tails (hydrophobic) – oriented
away from water water
Irving Langmuir, "The Constitution and Fundamental Properties of Solids and Liquids. II," Journal of the American Chemical Society 39 (1917): 1848-1906.
Lipid bilayer
1925 – Evert Gorter & F.
Grendel (University of Leiden, Holland)
• Compared the measured surface area of the erythrocytes and the surface area calculated from the lipid content of them.
• Gorter E, Grendel F. On Bimolecular Layers of Lipoids on the Chromocytes of the
Blood. J Exp Med. 1925 Mar 31;41(4):439-43.
Gortel, E. & Grendel, F. (1925) On bimolecular layers of lipoid on the chromocytes of the blood. J. Exp. Med. 41, 439–443.
14/11/2014
2
14/11/2014
Lipid bilayer
• twice as much lipid in the membrane of the red blood cells than needed for a monolayer → lipid bilayer
EC
Polar heads toward the intra- and extracellular space
IC
Gortel, E. & Grendel, F. (1925) On bimolecular layers of lipoid on the chromocytes of the blood. J. Exp. Med. 41, 439–443.
Apolar (hydrophobic)
tails in the middle
3
Gibbs free energy
[
Joule
]
G = H - TS
A spontaneous process is accompanied by a decrease in the Gibbs energy at constant
temperature and pressure.
At constant temperature and pressure the change in the Gibbs energy is equal to the maximum non-expansion accompanying a process.
work
14/11/2014
Hydrophobic interaction
• hydrophobic = water-repelling; low affinity (solubility) for water
• Walter Kauzmann (American chemist) - Nonpolar molecules in polar environment (solvents) are trying to minimize their contact with water
• 1 ”cage” formation → 2 clustering
• Factors affecting the strength of hydrophobic interaction
– Temperature (T
↑ ⇒ Strength
↑
)
– Number of carbons in the hydrophobic molecule (Length
↑ ⇒
Strength
↑
)
– Number of “non single” bonds (e.g. double, triple bonds…) in the hydrophobic molecule
(shape) ( # “non single” bonds
↑ ⇒
Strength
↓
)
4
H
2
O hydrophobic molecule
Thermodynamic changes
H
2
O
+
hydrophobic molecule
→
H
2
O H
2
O hydrophobic molecule hydrophobic molecule
Cage formation
(no interaction between hydrophobic molecules)
∆
H = small positive
∆
S = large negative
∆
G = positive
N ON SPONTANEOUS PROCESS
Clustering
(forming hydrophobic interactions)
∆
H = small positive
∆
S = large positive
∆
G = negative
SPONTANEOUS PROCESS
„Fluid mosaic” model
• 1972 - Singer and Nicholson „fluid mosaic” model
• phospho-lipid bilayer
• Fluid – lateral movement of the components („floating”)
• Mosaic – the mosaic-like arrangement of the macromolecules http://www.molecularexpressions.com/cells/plasmamembrane/plasmamembrane.html
Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(23):720-31.
14/11/2014
5
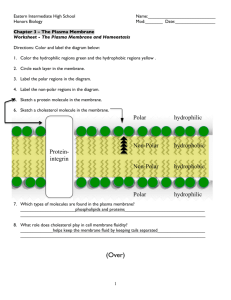
Structure of the cell membrane
Flip-flop
Lateral diffusion
Phospholipide molecule (~40-60%)
Polar
(hydrophilic) head
Non-polar
(hydrophobic) tail
~ 5 nm rotation
Protein molecule (~30-50%)
Functions of the membrane poteins
• Ion channels (Na + /K + ATPase; K + channel…)
• Transporters (Aquaporin-H
2
O transport)
• Structural elements
• Intracellular connections (anchoring – cytoskeleton)
• Extracellular connection (gap junction: cell to cell contact between cardiac cell)
• Signal transduction (action potential)
• Receptors (insulin receptor)
14/11/2014
6
The main components of the intra- and extracellular space
• water
• Ions
– Kations (K + , Na + , Ca 2+ )
– Anions (Cl , H
2
PO
4
− and HPO
4
2− ions)
• proteins
– Mainly intracellular localisation
– Negatively charged polyvalent (having more than one valence) macromolecules (pH! – isoelectric point)
14/11/2014
Membrane potential
-100 mV > U resting
< -30 mV
The electrical potential difference (voltage) across a cell's plasma membrane.
Microelectrode
0V
Intracellular space
Extracellular space
7
Ionic concentrations inside and outside of a muscle cell
Na + : 120 mM
K + :
2.5
mM
Cl : 120 mM
Na + :
20 mM
K + : 139 mM
Cl :
3.8
mM
14/11/2014
Forces controlling the movements of charged particles
Chemical potential energy:
The chemical potential of a thermodynamic system is the amount of energy
(Joule) by which the system would change if an additional particle were introduced ( ~ number of the particles!
).
Concentration gradient
→ diffusion : moving the particles through the permeable membrane from a high concentration area to a low concentration area
→ diffusion potential .
Energy: Capacity for doing work.
8
Electric potential energy
• the result of conservative Coulomb forces
• associated with the configuration of a particular set of point charges within a defined system
• work required by an electric field to move electric charges (Joule).
•
Electrical gradients: The sum of the “+” and “-” are not the same at the different points in space.
•
An electric field creates a force that can move the charged particles (the work of the electric field)
→ moving charged particles = electric current.
K + :
100 mM
Cl :
100 mM
K + :
5 mM
Cl :
5 mM
Force controlling the movements of ions through the cell membrane
Electro-chemical potential
= the combination (sum) of the chemical and the electric potential energy.
14/11/2014
9
Bernstein’s potassium hypothesis (1902)
Julius Bernstein (1839 - 1917) - German physiologist
1./ The cell membrane is selectively permeable to potassium
• Ca 2+ sensitive potassium channels
• Inwardly rectifying potassium channels
• Voltage-gated potassium channels
• “Tandem pore domain potassium channel” – “leak channel” (K2p)
1952: Hodgkin and Huxley suggested the leakage of current etchum, KA; Joiner, WJ; Sellers, AJ; Kaczmarek, LK; Goldstein, SA. (1995) A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature, 376 (6542): 690-5.
2./ The intracellular potassium cc. is high
3./ The extracellular potassium cc. is low
Bernstein,J.(1902).Untersuchungen zur Thermodynamik der bioelektrischen Strome. Pflugers Arch.ges. Physiol. 92, 521–562.
Bernstein’s potassium hypothesis
14/11/2014
K + : 100 mM
K + :
5 mM
Cl : 100 mM
Cl :
5 mM
10
Bernstein’s potassium hypothesis
The side with high concentration of positive ions becomes the negative side !!!!
electric gradient
(electrical potential)
+
[K + ]
[ Cl -
] [ Cl ]
[K + ]
K + gradient (chemical potential)
14/11/2014
How is it possible to quantify the
Bernstein’s hypothesis ?
(calculating the electrical potencial (value, number)
11
Walther Hermann Nernst
German physical chemist
(June 25, 1864 – November 18, 1941)
Calculating the electrical potential at which there is no longer a net flux (movement) of a specific ion across a membrane.
14/11/2014
Chemical potential energy
⇒
W chem
=NRT ln
N = number of moles associated with the concentration gradient
R = gas constant
T = absolute temperature
X
1
/ X
2
= concentration gradient
Electric potential energy ⇒ W electr
=NZFE
N = number of moles of the charged particles z = valency (number of + or – charges (e.g. K + : monovalent))
F = Faraday’s number (constant)
E = strength of the electric field = electric potential or electrostatic potential
= The work needed to move a unit electric charge from one point to another against an electric field (Joule/Coulomb = Volt).
12
Equlibrium (resting) condition
Electrical potential energy
NzFE
=
NRT ln
X
1
X
2 zFE
=
RT ln
X
1
X
2
Chemical potential energy
E
=
RT ln zF
X
X
2
1
Equlibrium potential
Nernst equation: What membrane potential
(E) can compensate (balance) the concentration gradient (X
1
/X
2
).
E
=
RT zF
X ln
X
2
1
The inward and outward flows of the ions are balanced
(net current = zero → equilibrium = stable, balanced or unchanging system).
14/11/2014
13
Nernst equation
E mV
=
RT X
1
E
= ln z zF
−
58 log
X
2
( )
( )
Ionic concentrations inside and outside of a muscle cell
Na + : 120 mM
K + :
2.5
mM
Cl : 120 mM
Na + :
20 mM
K + : 139 mM
Cl :
3.8
mM
[K + ]
[Na +
[Cl ]
]
⇒
E mV
⇒
E mV
⇒
E mV
= -58/1 log (139/2.5) = - 101.2 mV
= -58/1 log (20/120) = + 45.1 mV
= -58/1 log (3.8/120) = + 86.9 mV
= 30.8 mV E mV
=-92mV
14/11/2014
14
What happens if the cell membrane is not permeable to a charged component?
Frederick George Donnan
(1870-1956; Irish chemist)
Donnan equilibrium : characterising the equlibrium situation when the membrane is not permeable for some ionic components .
- non-moving charged component (e.g. intracellular proteins )
→ equlibrium concentration is different
more than one diffusible ion (K + , Cl )
14/11/2014
15
Donan equlibrium - at equlibrium
A
[K + ]
[
Cl -
]
[
Pr -
]
-
B
[K + ]
[
Cl -
]
+
Cl concentration gradient
Cl electrical gradient
K + electrical gradient
K + concentration gradient
Donnan rule of equilibrium
• Diffusible ions: K + , Cl -
• In equlibrium the elektro-chemical potentials are equal.
RT zF ln
[ ]
[ ]
=
E
=
RT zF ln
[
Cl out
[ ]
]
[ ]
K in [ ]
=
[
Cl out [ ]
]
[ ][ ] [ ][
Cl out
]
The Donnan rule is valid only when the ions are passively distributed.!
The Gibbs–Donnan equilibrium is a phenomenon that contributes to the formation of an electrical potential across a cell membrane.
14/11/2014
16
What happens if the Donnan rule is not obeyed?
Goldman-Hodgkin-Katz Constant field equation (Goldman equation)
David E. Goldman (USA)
Alan Lloyd Hodgkin (England)
Bernard Katz (England).
To determine the potential across a cell's membrane taking into account all of the ions with different permeabilities through the membrane.
14/11/2014
17
Goldman equation
The Goldman equation for M positive ionic species and A negative:
E m
=
RT
F
∑ N i ln
∑ N i
P
M
+ i
P
M
+ i
M i
[ ] in out
+
+ ∑ M j
∑ M j
P
A
P
A
− j
− j
A j in
[ ] out
•E m
•P ion
= The membrane potential
= the permeability for that ion
•[ion] out
•[ion] in
= the extracellular concentration of that ion
= the intracellular concentration of that ion
•R = The ideal gas constant
•T = The temperature in Kelvins
•F = Faraday's constant
A "Nernst-like" equation with terms for each permeant ion (permeability).
- All the ions are involved with different concentrations.
- Good agreement with the measured values (muscle cell: U measured
=-92mV_U calc.
=-89.2mV).
Goldman equation
The membrane potential is the result of a
„compromise” between the various equlibrium
potentials, each weighted by the membrane
permeability and absolute concentration of the ions.
14/11/2014
18
The end!
14/11/2014
19