IODOMETRY
advertisement

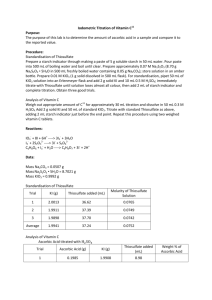
Experiment No. ___________________ Date ___________________ IODOMETRY AIM To determine the amount of copper by a Redox Titration. INTRODUCTION Iodide ion (I-) is a moderately effective reducing agent that has been used for the analysis of oxidants. The lack of a good method of end point detection makes direct titration of oxidizing agents by solutions of iodide salts impractical. Thus, indirect procedure is always employed. This involves reduction with a moderate unmeasured excess of potassium iodide. Liberated iodine (I2) is then titrated with a standard solution of a reducing agent. The quantity of the iodine is equivalent in quantity to the oxidant being determined. Sodium thiosulfate, Na2S2O3, is mostly used for this purpose. The reaction between iodine and thiosulfate ion is as follows: 2S2O32- + I2 ↔ S4O62- + 2IThe end point in the titration is readily established by means of starch solution. It should be emphasized that starch is partially decomposed in the presence of a large excess of iodine. For this reason the indicator is never added to an iodine solution until the bulk of that substance has been reduced. The change in color of the iodine from a red-brown to a faint yellow signals the proper time for the addition of the indicator. REAGENTS AND APPARATUS CuSO4.5H2O (unknown solution, already prepared) Sodium thiosulfate, Na2S2O3.5H2O (0.5 L of 0.1 M for 3 students) Sodium carbonate, Na2CO3 (in the balance room) Potassium iodate, KIO3 (primary standart) Potassium iodide, KI (in the balance room) Potassium thiocyanate, KSCN (in the balance room) Hydrochloric acid, HCl (25 mL of 6.0 M for 2 students) Sulfuric acid, H2SO4 (25 mL of 3.0 M for 2 students) Starch indicator (already prepared) 250mL conical flasks 100 mL graduated cylinder buret PROCEDURE A) Preparation of Na2S2O3 Solution 1) 2) Heat 0.5 L of distilled water to boiling in a beaker covered with a watch glass. Boil for at least 10 minutes. Cool and add necessary amount of Na2S2O3.5H2O to prepare 0.5 L of 0.1 M Na2S2O3 and add 0.05 g of Na2CO3. 3) Thiosulfate solution is readily effected by microorganisms and its concentration is changed. This effect can be minimized by addition of several substances like Na2CO3, NaOH, borax (NaB2O4) and chloroform (CHCl3). Stir until Na2S2O3.5H2O and Na2CO3 are dissolved. B) Standardization of Na2S2O3 Solution 1) 2) 3) 4) Weigh 0.12 to 0.15 g samples of KIO3 into 250 mL erlenmeyer flask. Dissolve in 50 mL of water and add 2.0 g of KI After KI salt has dissolved, add 2.0 mL of 6.0 M HCl and titrate immediately with Na2S2O3 solution until the color of the solution becomes pale yellow. Add 5.0 mL of starch indicator and titrate to the disapperance of the blue color. The reactions involved can be expressed as follow: IO3 5I 6H 3I2 3H2O 2 6I S O2 3I2 6S2O3 4 6 2 3H O IO3 6H 6S2O2 I 3S4 O6 2 3 5) Two replicates will be performed for each student. Calculate the molarity of the thiosulfate solution for the results of 4 students. C) Determination of Copper 1) 2) 3) Take your unknown sample into a 250 mL erlenmeyer flask and add 50 mL distilled water. Add 1.0 mL of 3.0 M H2SO4 and then 4.0 g of KI. Titrate immediately with standardized Na2S2O3 solution until the solution becomes pale yellow and then, add 2.0 g of potassium thiocyanate, KSCN and 5.0 mL of starch indicator. It has been found experimentally that the titration of iodine by thiosulfate in the presence of CuI tends to yield slightly low results because appreciable quantities of iodine are adsorbed on the solid. This difficulty is largely overcome by the addition of thiocyanate (SCN-) ion which also forms a sparingly soluble copper (I) salt. Part of the copper (I) iodide is converted to the corresponding thiocyanate at the surface of the solid: 2Cu2 4I 2CuI(s) I2 CuI(s) SCN CuSCN(s) I Accompanying this reaction is the release of the adsorbed iodine, I2, thus making it available for titration. Early addition of SCN- must be avoided, however, because of the tendency for that ion to reduce iodine slowly. 4) 5) Swirly vigorously for several seconds. Continue the titration with vigorous mixing until the blue starch/iodine color is decolorized and does not return for several minutes. The reactions involved can be expressed as follow: 2Cu 2 4I 2CuI(s) I2 2 2I S O2 I2 2S2O3 4 6 2 2CuI(s) S O2 2I - 2Cu 2 2S2O3 4 6 6) Report the result in terms of mg Cu in your unknown sample. PRE-LAB STUDIES Read pages 512-514 (20B-2) from the textbook (9th Ed) 1) Why KMnO4 is preferred as an oxidizing agent in red-ox titrations? 2) Why do we apply an indirect method in iodometry? 3) What is the function of starch in this experiment and why do not we add it at the beginning of the titration? 4) What is the name of primary standard used in the standardization of thiosulfate solution? 5) What is the reason of rarely using iodine as primary standard for the standardization of thiosulfate solution? POST-LAB STUDIES 1) 2) 3) 4) During the preparation of sodium thiosulfate solution, i) why do we add Na2CO3? ii) why should we store Na2S2O3 solution in dark and closed bottles? Answer the following questions for the standardization of sodium thiosulfate: i) Explain what happens during the titration and write the related reactions. ii) Why should the medium be acidic? iii) What kind of error (positive/negative) is expected to arise if the titration is not started right after the addition of acid? What is the function of KSCN in the Cu2+ determination? Write the related reaction(s). Why do not we add KSCN at the beginning of the experiment? What kind of error is obtained if it is not added during the titration? Explain. Name surname: Section: Date: REPORT SHEET FOR IODOMETRY B. Standardization of Na2S2O3 Solution Student 1 Student 2 Student 3 Mass of Volume of Concentration of KIO3, g Na2S2O3, mL Na2S2O3, mol/L Concentration of Na2S2O3, mol/L ̅ 𝐬) (𝐗 Replicate 1 Replicate 2 Replicate 1 Replicate 2 Replicate 1 Replicate 2 C. Determination of Copper Volume of Na2S2O3, Mass of Mean mass, True mass of % Relative mL copper, mg ̅ 𝐬) mg (𝐗 copper, mg Error Replicate 1 Replicate 2 TA`s Name and Signature: