Reactivity of Metals in Single Replacement Reactions
advertisement

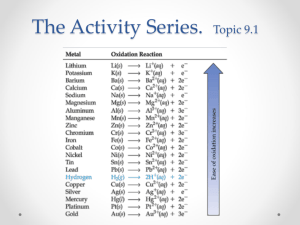
Activity Series 1 Name ______________________________________________ Date _________ Period ______ Reactivity of Metals in Single Replacement Reactions Purpose After reading the lab, create your own purpose below: ______________________________________________________________________________ ______________________________________________________________________________ Pre-Lab Discussion (Do not copy in your lab book) Some metals are more reactive than others. In a single replacement reaction, the more active metal will end up bonded with a nonmetal or polyatomic ion, while the less active metal will be found as an elemental solid. The activity series (which includes metallic hydrogen) orders the metals in terms of reactivity. This lab should show you how the activity series was determined through experiment, and that some single replacement reactions do not occur. Materials Beakers (100 mL) Pipets Scoopula Well Plate Cu (s) Mg (s) Fe (s) Zn (s) 3.0 M HCl 0.1 M Cu(NO3)2 0.1 M Mg(NO3)2 0.1 M Fe(NO3)3 0.1 M Zn(NO3)2 Procedure 1. Place the well plate on top of a paper towel and label the rows and columns from Table 1 on the paper towel. Leave the wells that are blacked out in Table 1 empty. 2. Using the scoopula, place a small amount (2-3 pieces) of Copper in the top row of the well plate. 3. Repeat step 2 for Magnesium (1 strip of metal), Iron (1 microspatula), and Zinc (1 piece of metal). 4. Using the pipet, add enough Cu(NO3)2 to cover the metals in column 1. 5. Repeat step 4 for the other solutions. Allow 2-3 minutes for signs of a chemical reaction to appear and record observations in Table 1. 6. Using a new pipet, draw the liquid out of each well. Observe the solid left behind and record any new observations in Table 1. Squeeze the pipet out into the sink and repeat for the remaining wells. Activity Series 2 Data Table 1 – Reaction Observations Cu(NO3)2 Mg(NO3)2 Fe(NO3)3 Zn(NO3)2 HCl Cu Mg Fe Zn Questions 1. Using examples, describe the signs of chemical reactions you observed in the lab. 2. Which of the metals reacted with the most compounds? Which reacted with the fewest? 3. Which of the metals you tested were able to replace the hydrogen in a reaction with hydrochloric acid? What do you think is a product of the reactions that occurred? 4. Based on your observations, why is copper used to make jewelry? 5. Write balanced equations for all of the reactions you determined had occurred in lab. (Note: Use Fe3+ for iron ions and Cu2+ for copper ions) Conclusion Using your data, create and justify an activity series (most reactive to least reactive) for the metals you tested. Include hydrogen in your list. Compare your activity series to the one to the right. Are they similar? Explain. Activity Series of Metals Li K Ba Sr Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H2 Cu Hg Ag Pt Au