Lab XII - Single Replacement Reactions
advertisement

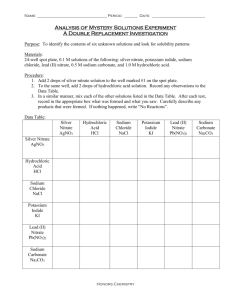
Single Replacement Reactions Name:_______________ Period:____ PURPOSE: To observe and practice writing down molecular, complete ionic, and net ionic equations for single replacement reactions. THEORY: Most reactions in chemistry can be classified as one of two kinds: double replacement (metathesis) reactions or single replacement reactions. In a double replacement reaction, typically two aqueous solutions containing ionized or dissociated species are mixed. Consequently ions in the solutions are brought into contact with one another, with the possibility of creating a new compound that is either water (an acid base reaction), a gas, or an insoluble salt. Single replacement reactions typically occur when a solid metal is brought into contact with an aqueous solution of another metallic salt. Depending on the activity, or the relative ease with which the metal gives up its electrons, it can transfer electrons to the cation of the metal in solution, resulting in the formation of a new metallic solid, and the consequent dissolution of the original metal. Single replacement reactions can also occur with the hydrogen ion, H+(aq) that can be donated from an acid such as HCl, or even water if the metal has a high enough activity. Finally, the halogens can also be involved in a series of single replacement reactions, with the relative activity being easily deduced from the position of the halogens on the periodic table. Before one can understand why chemical reactions occur, one must gain experience predicting, observing, and recording the results of many different reactions. Typically there are three kinds of equations that can be written down for a chemical reaction: 1) The molecular equation, in which all species are written together as formula units or molecules, but the dissociation or ionization of compounds is not explicitly noted. 2) The complete ionic equation, which directly illustrates which species are ionized or dissociated in solution. 3) The net ionic equation, which allows one to quickly determine what occurred in the reaction, as it is created by deleting all spectator ions from the complete ionic equation. Today you will observe many chemical reactions and practice writing down these equations for each one. MATERIALS: 1) M solutions of the following: Barium chloride Potassium iodide Copper(II) sulfate Zinc nitrate Magnesium nitrate Sodium bromide Silver nitrate Sodium phosphate 1.0 M solutions of the following Sodium carbonate Hydrochloric acid Lead(II) nitrate Copper(II) nitrate Potassium chloride Solid samples of: Copper Magnesium Zinc Silver Sodium 24 well plate (see figure below) Plastic Beral Pipets 1% Phenolphthalein solution Schematic Diagram of 24-well Plate (shown with where each metal is placed) 1 A 2 Copper 3 4 5 6 Copper Copper Copper copper Mg Mg Mg Mg Zn Zn B Mg C Zn Zn Zn D Ag Ag Ag Ag Ag PRELAB: All parts must be completed before you can begin the lab. Copy the following tables exactly into your lab book. Use a minimum of a full page for Table I Data Table I – Single Replacement Reactions: Well A2 A3 A4 A5 A6 Reactants Solid copper and Magnesium nitrate Solid copper and Sodium nitrate Solid copper and Zinc nitrate Solid copper and Silver nitrate Solid copper and Hydrochloric acid Observations B1 B3 B4 B5 B6 C1 C2 C3 C5 C6 D1 D2 D3 D4 D6 Solid magnesium and Copper(II) nitrate Solid magnesium and Sodium nitrate Solid magnesium and Zinc nitrate Solid magnesium and Silver nitrate Solid magnesium and Hydrochloric acid Solid zinc and Copper(II) nitrate Solid zinc and Magnesium nitrate Solid zinc and Sodium nitrate Solid zinc and silver nitrate Solid zinc and hydrochloric acid Note: The D wells will be Solid silver and Copper(II) nitrate Solid silver and Magnesium nitrate Solid silver and Sodium nitrate Solid silver and Zinc nitrate Solid silver and hydrochloric acid Done by the instructor Data Table II – The Reaction of sodium with water Experiment One Reactants Solid sodium added to water Two Solid sodium added to water with phenolphthalein Observation CALCULATIONS: Single Replacement Reactions and the reactions of sodium. For each reaction that occurred, write down the molecular, complete ionic and net ionic equations for the reaction. Clearly show in your calculation which reaction well you are discussing. For example, for well B1 you should write: Reaction for well B1: ME : Mg(s) + Cu(NO3)2(aq) Mg(NO3)2(aq) + Cu(s) CIE: Mg(s) + Cu2+(aq) + 2NO3-(aq) Cu(s) + Mg2+(aq) + 2NO3-(aq) NIE: Mg(s) + Cu2+(aq) Mg2+(aq) + Cu(s) Show this process for each reaction. If the same reaction occurred in more than one well, you may write the reaction once, and then refer to it for subsequent wells. If no reaction occurs, clearly state that. For example, if nothing happened in well C6, say Reaction for well C6: No Reaction, or NR Now look at your reactions. The solid metal that produced the greatest number of reactions is the most active. From your data produce your own clearly labeled activity series from the metals (Cu, Na, Mg, Zn, Ag) and the hydrogen ion. Compare your ordering to that of the activity series in the text, explaining any differences. Reactions of sodium: Write down the ME, CIE, and NIE for this reaction. DISCUSSION: Answer the following questions neatly and in complete sentences in your lab book. a) Explain the fundamental difference between single and double replacement reactions. b) Recall we discussed the white coating that is formed on the sodium metal as it stands in air. Write a balanced chemical equation for this reaction, and state what kind of reaction this is. c) Explain why the solution turned pink when phenolpthalein was added to the reaction of sodium with water. What could be added to make the solution return to a clear color? d) Explain why one would never want to use water to put out a fire that involved an alkali metal. e) Why is sodium hydroxide the product of the reaction of sodium with water instead of sodium oxide? f) If one placed 25 g of sodium metal in water, how many grams of hydrogen gas would be made? Be sure to complete your lab with a meaningful conclusion! Procedure: Single Replacement Reactions A– Activity series of metals 1) Once again obtain a clean and dry well plate. 2) Place 20 drops of copper(II) nitrate into wells B1 and C1. 3) Place 20 drops of magnesium nitrate into wells A2, and C2 4) Place 20 drops of sodium nitrate into wells A3, B3, and C3. 5) Place 20 drops of zinc nitrate into wells A4 and B4 6) Place 20 drops of silver nitrate into wells A5, B5, C5, 7) Place 20 drops of hydrochloric acid into wells A6, B6, C6, 8) Insert one piece of copper metal into each filled well of the A row. 9) Insert one piece of magnesium metal into each filled well of the B row. 10) Insert one piece of zinc metal into each filled well of the C row. 11) The instructor will have a well set up showing the reaction of silver metal with each solution. Make sure you look at this plate and record appropriate observations. 12) If needed, gently shake the well plate to submerge all of the metal. It is ok if a small amount of the metal rests above the solution. 13) Record observations once all metal pieces have been inserted, and then again after 5 minutes. At this time the instructor will do the sodium demo. After this is completed, make a final set of observations to see if anything has changed. 14) Dump the contents of all wells into the waste beaker provided. Use Q-tips to remove any pieces of metal stuck in the wells. Clean the plate with soap solution, tap water, and distilled water. Return to you desk for class discussion. F – The reaction of sodium metal with water. The instructor will demonstrate the reaction of sodium with water. Record observations for both demonstrations.