Sp2004 Org II Exam #1 Ch (14-17) (100 points) 1) Draw a Lewis
advertisement
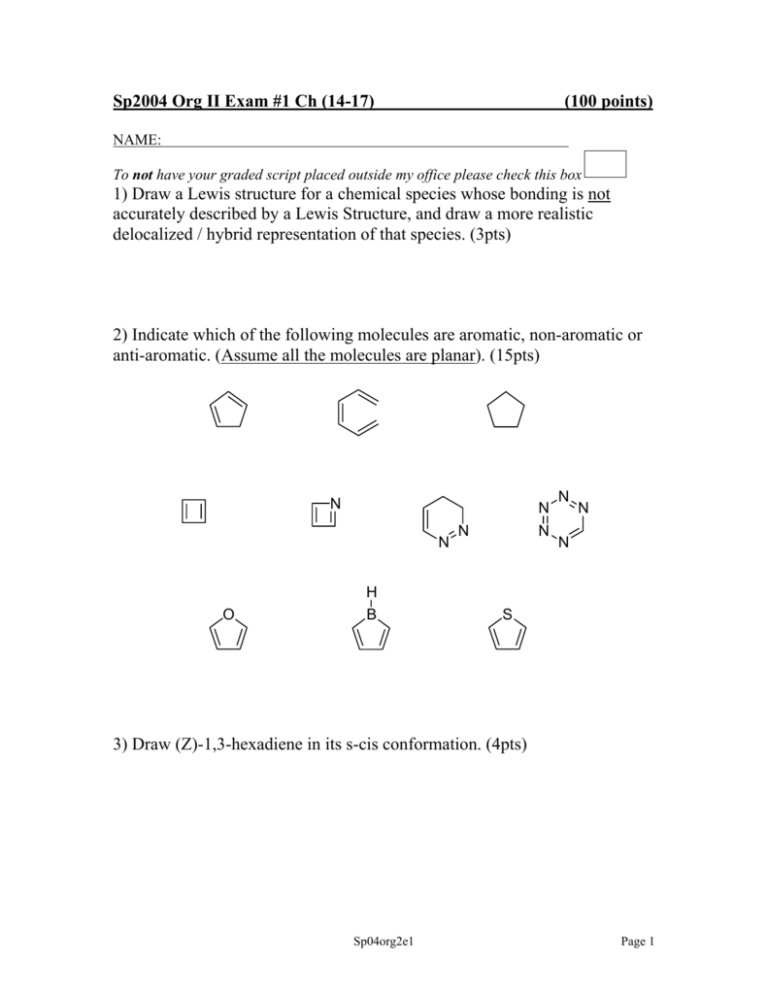
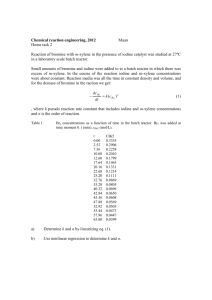
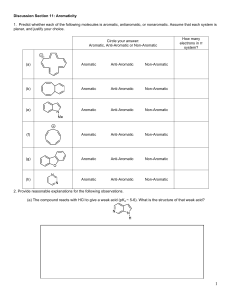
Sp2004 Org II Exam #1 Ch (14-17) (100 points) NAME: To not have your graded script placed outside my office please check this box 1) Draw a Lewis structure for a chemical species whose bonding is not accurately described by a Lewis Structure, and draw a more realistic delocalized / hybrid representation of that species. (3pts) 2) Indicate which of the following molecules are aromatic, non-aromatic or anti-aromatic. (Assume all the molecules are planar). (15pts) N N N O H B N N N N N S 3) Draw (Z)-1,3-hexadiene in its s-cis conformation. (4pts) Sp04org2e1 Page 1 4) By applying the polygon rule to the below cyclic hydrocarbon: a) draw out the MO energy level diagram b) label the MO’s using π1….π4* c) circle one pair of degenerate orbitals d) draw in the electrons and explain whether this compound is predicted to be aromatic or antiaromatic. (8pts) 5) The following compound was produced in a Diels-Alder reaction. CO2H CF3 How many carbon atoms are in this molecule? How many sp2 hybridized carbons are in this molecule? Draw the diene and dienophile which react together to give this product. (10pts) Sp04org2e1 Page 2 6) Predict the products in the following reactions (if you believe no reaction will occur, indicate this!), paying attention to regio/stereochemistry where applicable. (21pts) heat O NC CN HNO3, H2SO4 SO3H O H NaOCH3, CH3OH CH2CH3 H CH2CH3 H3C OH 1) NaOH 2) CH3CH2Br Br2, FeBr3 2 equivalents of mcpba CH2CH3 CH3Cl, AlCl3 Sp04org2e1 Page 3 7) When one equivalent of bromine is added to the following conjugated diene, a mixture of two products is formed. Br-Br Draw the two products and provide the step-by-step mechanism which explains the generation of both products. If the reaction temperature was reduced by 47oC, which product would increase in yield? (9pts) Sp04org2e1 Page 4 8) For both below substitution reactions: (i) draw the expected product (ii) explain why the bromine substitutes at that specific site for each reaction. (10pts) CH3 Br2, uv light Br2, FeBr3 F3C Sp04org2e1 Page 5 9) Please fill in the blanks (reagents and compounds). (18pts) Br NO2 PhO O CH2CF2H excess HBr Cl NO2 O C PhCH2C6H5 heat Br Sp04org2e1 Page 6 10) What is the definition of a Pericyclic reaction? (2pts) *Bonus question* (up to 3pts) Draw BENZYNE, and explain why it is aromatic. Sp04org2e1 Page 7