Why Use A Barge?

Why Use A Barge?
SUBJECT: Science
GRADE LEVELS: 6-7-8
STATE STANDARDS: 11.A.3a,
11.A.3b
11.A.3c
Objectives:
Students will understand that the structure of a barge allows it to carry a greater amount of freight.
Materials Included:
Copies of Student Activity Sheets #1 and #2 for each student (attached)
Additional Materials Needed:
2 eight-inch square pieces of aluminum foil a dish pan plastic shoe box or 13 x 9 inch pan half filled with water approximately 200 paper clips paper towels.
Warm-up/Discussion:
Ask students if they have ever seen a vessel carrying goods along a canal. If they have, ask them to describe the vessel.
Ask them how it was different from other boats. Students should understand that barges are flatbottomed vessels. Barges also do not have motors or other means of propelling themselves. They must be pushed or pulled by some other means.
Today, boats do this pushing or pulling. In the early days of the Canal, mules or horses pulled the barges. If students have not seen a barge, have them refer to the illustration on Student Activity
Sheet #2.
Learning Activities
Divide students into groups of three. Tell them that they are going to carry out an experiment that will help them to understand why barges are flat-bottomed. Distribute the materials listed above to each student group.
Instruct the students to build a model of a barge and a vessel with a bottom which is deeper in the center than at the sides. Again refer students to the illustrations if they are having difficulty. Allow the students to try different folding and construction methods. Student will need only the foil to build each model; they should not use staples, tape or other items. When students have completed the two models ask them to predict which vessel will hold the most paper clips without sinking.
Have them record their predictions on Student Activity Sheet #1.
One at a time, have the students place each vessel into their pan of water and carefully add single paper clips until it sinks. Remind students to keep a count of how many paper clips each model holds. Be prepared, the barge will hold a very large number a paper clips! Students will have to dry the paper clips after the first test to be re-used on the second model. You may want to have some extra clips on hand in case you have some expert barge builders!
After each group has completed the experiment, ask students to share their results. The barge should have held more paper clips than the other model. If students had different results discuss the variables which may have caused this.
Have students complete the questions on their sheets. Discuss why barges are used to carry goods on the canal.
Assessment:
Student Activity Sheet #1 can be collected and graded. Answers will vary.
Ideas for Additional Activities:
Have students construct vessels of other shapes and test them to see how much weight they can hold.
If possible, visit a site where students can watch barges on a canal. Have students observe and record the barges they see and the freight which the barges carry.
Name That Stone
SUBJECTS: Science
GRADE LEVELS: 6-8
STATE STANDARDS: 11.A.3a,
11.A.3b, 11.A.3c, 13.A.3c,
13.B.3f
Objectives:
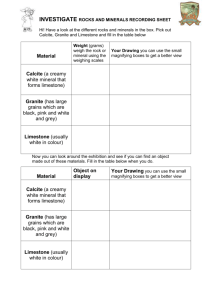
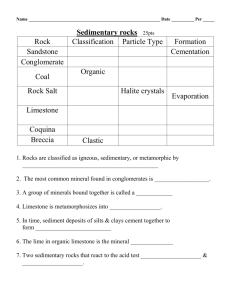
Discuss the importance of limestone both as the underlying rock for most of the I & M Canal Corridor and its significance as a building material in communities along the Canal.
Identify chemical & physical characteristics of limestone.
Materials Included:
Copies of Student Activity Sheet s for each student
Additional Materials Needed:
small pieces of limestone white vinegar small cups
Vocabulary:
calcium carbonate glaciers carbon dioxide
Warm-up/Discussion
Distribute copies of Student Sheet #1 .
Have students read the background information on limestone.
Learning Activities
Divide the class into groups of three or four students each. Distribute the materials each group will need a piece of limestone and a small cup of vinegar.
Have students carry out the experiment described on the student sheet. Make sure they record their observations. Discuss their results. Explain that the bubbles they see are produced when the vinegar and limestone interact to create carbon dioxide
Ideas for Additional Activities:
Have students go on a rock walk on the school grounds or in the neighborhood. Instruct students to collect several different rock samples. Use the vinegar test to identify rocks that are composed of limestone.
Take students on a limestone hunt. Walk or take a school bus past various buildings in the community that are constructed of limestone. Ask students to describe various characteristics of the stone. Have them draw pictures of the buildings.
Invite a representative from a local quarry or a contractor who works with limestone to speak to the class.
Teacher Note: Over time, the calcium in limestone can be replaced by magnesium. When a sufficient amount of magnesium is present the rock is then called dolomite. Locally quarried stone is dolomite. For this reason you may not observe pronounced fizzing when your local stone to produce some observable, if slight, reaction. You may wish to order limestone samples from a scientific supply house to carry out the experiment.
Assessment:
Student Activity Sheet #2 can be collected and graded. Answers will vary.