Thermogenic brown and beige/brite adipogenesis in humans
advertisement

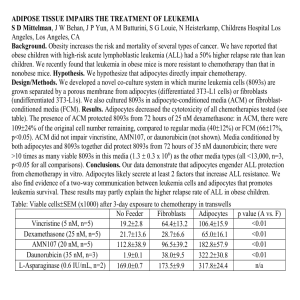
Annals of Medicine, 2014; Early Online: 1–9 © 2014 Informa UK, Ltd. ISSN 0785-3890 print/ISSN 1365-2060 online DOI: 10.3109/07853890.2014.952328 SPECIAL SELECTION: BROWN FAT Thermogenic brown and beige/brite adipogenesis in humans Rubén Cereijo, Marta Giralt & Francesc Villarroya Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. Departament de Bioquímica i Biologia Molecular, Institute of Biomedicine (IBUB), University of Barcelona, and CIBER Fisiopatología de la Obesidad y Nutrición, Barcelona, Catalonia, Spain Evidence from rodents established an important role of brown adipose tissue (BAT) in energy expenditure. Moreover, to sustain thermogenesis, BAT has been shown to be a powerful sink for draining and oxidation of glucose and triglycerides from blood. The potential of BAT activity in protection against obesity and metabolic syndrome is recognized. Recently, an unexpected presence and activity of BAT has been found in adult humans. Here we review the most recent research in this field and, specifically, how new findings apply to humans. Moreover, we seek to clarify the underlying biological processes occurring beyond the burst of new nomenclature in the field. The cell type responsible for thermogenesis, the brown adipocyte, arises from complex developmental processes. In addition to ‘classical’ brown adipocytes, present in developmentally programmed BAT depots, there are brown adipocytes, named ‘brite’ (from ‘brown-in-white’) or ‘beige’, which appear in response to thermogenic stimuli in white fat due to the so-called ‘browning’ process. Beige/brite cells appear to be important components of BAT depots in adult humans. In addition to the known control of BAT activity by the sympathetic nervous system, metabolic and hormonal signals originating in muscle or liver (e.g. irisin, FGF21) are recognized as activators of BAT and beige/brite adipocytes. Key words: Antiobesity agents, brown adipose tissue, metabolic syndrome X, obesity, white adipose tissue Introduction: the shifting history of human brown adipose tissue There is a long history of evidence for the existence of brown adipose tissue (BAT) in humans. Classical anatomists and histologists reported the presence of masses of brown adipose tissue containing the typical multilocular adipocyte morphology in several anatomical sites in humans. The prevalence of BAT depots in human neonates and the progressive decrease in the amount of BAT in adults were commonly recognized, and it was assumed that BAT progressively involutes with age. Given the recognized role of BAT as a thermogenic tissue, it was also assumed that BAT function in humans was probably restricted to the neonatal period, during which it would provide the heat needed to cope with the thermal stress associated with birth (1). Key messages • Thermogenic, energy-dissipating, beige/brite adipocytes are a subtype of brown adipocytes with a cell lineage origin distinct from ‘classical’ brown adipocytes. • Human adult adipose tissue possesses a remarkable plasticity and may contain thermogenic, energydissipating, beige/brite adipocytes. • The energy-dissipating and metabolite oxidation properties of beige/brite adipocytes (like ‘classical’ brown adipocytes) make them attractive candidates to be stimulated in order to prevent obesity and ameliorate hyperglycemia and hyperlipidemia. However, studies in rodents reported by Rothwell and Stock in the late 1970s proposed that BAT was not only a site of coldinduced thermogenesis but was also a main site of diet-induced thermogenesis (2), reigniting interest in the role of human BAT owing to its obvious potential to promote energy expenditure and protect against obesity. The subsequent discovery of UCP1 (uncoupling protein-1) in rodents as the key protein that conferred specific thermogenic properties to BAT mitochondria was soon followed by the identification and characterization of human UCP1, confirming that the molecular mechanisms necessary for BAT-associated thermogenesis were present not only in rodents but also in humans (3). However, skepticism about the physiological relevance of BAT in humans prevailed (4). Nevertheless, some lines of evidence continued to provide support for the physiological role of BAT, including genetic association studies that related polymorphisms of the UCP1 gene with altered energy balance and metabolism in adult human patients. A single-base polymorphism (A-3826G) in the 5’ regulatory region of the UCP1 gene is relatively frequent in the human population. Multiple studies in the late 1990s reported significant associations of this polymorphic variant with metabolic abnormalities in distinct adult human populations. After reviewing 12 independent reports, Gonzalez-Barroso et al. (5) concluded that the A-3826G polymorphism is not a major contributor to obesity development, but it is related to an increased propensity toward Correspondence: Francesc Villarroya, Departament de Bioquimica i Biologia Molecular, Facultat de Biologia, Universitat de Barcelona, Av. Diagonal 643, 08028-Barcelona, Catalonia, Spain. E-mail: fvillarroya@ub.edu (Received 6 June 2014; accepted 4 August 2014) Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. 2 R. Cereijo et al. weight gain over time, especially in persons with a higher risk of obesity. Since that time, more than 20 additional studies of distinct adult human populations have confirmed the association of this UCP1 gene polymorphism with metabolic and energy balance alterations in human adults. Of course, these genetic data could be interpreted as evidence for a biological role of UCP1 activity in neonatal BAT that has consequences in adulthood, but the possibility remained that UCP1-related BAT activity was physiologically relevant in adults. The concepts governing thinking on the subject of BAT in human adults have recently undergone a radical shift, propelled initially by the serendipitous finding of metabolically active ‘adipose tissue’ in patients suspected of cancer—a finding made possible by the introduction of [18F]-fluorodeoxyglucose-based positron emission tomography (PET) assays for diagnostic purposes. Active metabolic sites revealed by PET, unrelated to tumors, were found mainly in neck and shoulder areas of adults (6). Three independent studies in 2009 demonstrated that these sites corresponded to metabolically active BAT, leading to the current concept that the majority of human adults possess metabolically active BAT (7–9). Notably, recent studies that have assessed the association of UCP1 -3826A/G and PET-determined BAT activity established an association between this UCP1 gene polymorphism and the extent of age-related decreases in BAT activity (10). In recent years, multiple studies have addressed studies on BAT activity in distinct human populations, confirming several conclusions: BAT activity is higher in women than men; BAT activity progressively decreases with age; BAT activity is induced by cold exposure; and BAT activity is reduced in obese patients. Recent reviews provide updated information on the PET scan-based assessment of BAT activity in humans (11,12). Beige/brite: nomenclature, tissues, and cellular identities The story of the recent rediscovery of BAT in adult humans has coincided with important advances in our understanding of the cell biology of the brown adipocyte and a growing awareness of its unexpectedly high degree of complexity. In rodents, classical anatomical brown fat depots (i.e. interscapular) are present throughout life. BAT develops during fetal life, whereas white adipose tissue (WAT) grows mainly after birth. However, this apparent transformation of adipose depots that occurs in mice after birth, from predominantly brown to predominantly white, does not rely on the conversion of mature brown adipocytes to white adipocytes, but is instead based primarily on the differentiation of white adipocytes from precursor cells (13). During embryonic development, adipocytes develop from mesenchyme of mesodermal origin, with the exception of the mesenchyme in the cephalic region, which has an ectodermal origin (14). Numerous studies have sought to establish the identity of adipocyte precursor cells (15). It is now well established that brown and white adipocytes have distinct developmental origins. At least two distinct adipocyte lineages have been defined based on expression of the myogenic lineage marker Myf5 (myogenic factor 5). Brown adipocytes present in BAT depots originate from Myf5-expressing precursors, which are also the source of skeletal myogenic cells and a subpopulation of white adipocytes (16–18). In contrast, most white adipocytes are derived from Myf5-negative precursors; this also appears to be the case for brown adipocytes that develop in WAT as a consequence of the ‘browning’ process (16,19,20). The so-called browning of WAT is defined as the appearance of functional brown adipocytes—also named ‘brite’ (‘brown-in-white’) or ‘beige’ (see below)—in WAT depots after thermogenic stimuli. In rodents, it is now well established that this process, together with the ‘recruitment’ of existing BAT depots, is required for optimal adaptive energy expenditure (21). As discussed below, cold exposure and adrenergic signaling are the main inducers of these processes, although other factors, shared to varying degrees, might also contribute. Increased browning of WAT is also often found in genetically engineered rodent models resulting in suppressed ‘classical’ BAT activity (22), possibly as a compensatory process. Adipocyte cells were formerly classified into two types: unilocular, fat-storing, white adipocytes; and multilocular, thermogenic brown adipocytes. The current classification scheme now includes a third category of adipocytes, the so-called beige or brite adipocytes, also sometimes referred to as ‘inducible’ brown adipocyte-like cells (23–25). These cells have phenotypic and functional characteristics of brown adipocytes, but are found in anatomical white adipose depots. In contrast, brown adipocytes located in defined anatomical BAT depots are often referred to as ‘classical’, ‘constitutive’, or ‘developmentally programmed’ brown adipocytes. This terminology is clearly applicable in rodents, but, as described below, its application to adipocyte cell biology in humans is not consolidated yet. There is genetic evidence that the capacity to induce the appearance of beige/brite adipocytes in WAT depots is highly relevant for protection against obesity in rodents. Accordingly, differences in beige/brite adipocyte abundance in WAT reported between mice strains correlate positively with their resistance to induce obesity by diet (26). There are also significant differences in the number of these beige/brite cells between WAT depots; they are most abundant in subcutaneous inguinal WAT and least abundant in visceral perigonadal WAT (27). Recent lineage-tracing studies using transgenic mice indicate that beige/brite adipocytes arise from a cell lineage (Myf5negative) different from that leading to brown adipocytes (Myf5-positive) in anatomically defined BAT depots (16). However, research to clarify the precise origin of distinct cells found in WAT—genuine white adipocytes and beige/brite adipocytes—remains ongoing. In fact, whether beige/brite adipocytes derive from pre-existing white adipocytes through a process of transdifferentiation or through de novo adipogenesis from a specific subgroup of precursor cells remains a matter of debate (28,29). These processes are not mutually exclusive, and it is possible that their relative importance in different WAT depots may vary. Indeed, it has been proposed that β3-adrenergic activation induces browning in epididymal WAT through proliferation and further differentiation of precursors, whereas in inguinal WAT it acts through white-to-brown transdifferentiation (19). Support for the white-to-brown adipocyte transdifferentiation process was first proposed based on the observed lack of induced cellular proliferation and the presence of morphological intermediate forms of adipocytes (‘paucilocular’) in the transition from large unilocular white adipocytes to UCP1positive multilocular beige/brite adipocytes in response to cold exposure or β3-adrenergic receptor stimulation (30–32). Recent lineage-tracing experiments in adult mice directly demonstrated that mature white adipocytes in inguinal WAT have the potential to convert to beige/brite adipocytes and, furthermore, that this is a reversible process that depends on environmental temperature (33). However, another recent study in mice using a pulse-chase fate-mapping technique to mark mature white adipocytes reached the conclusion that most beige/brite adipocytes arise from precursor cells during browning in inguinal WAT (34). Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. Brown and beige/brite adipogenesis in humans 3 Another question is whether there are specific precursor cell types for beige/brite adipocytes distinct from those for white adipocytes in WAT. As discussed below, gene-profiling analyses of primary WAT preadipocytes differentiated in vitro have suggested two types of preadipocytes (25). In contrast, in vivo mouse studies have been less conclusive, due in part to the difficulty of defining a true white adipocyte specific marker, or even unique gene markers for tracing WAT adipocyte developmental origins. So, the actual identity of beige/brite precursor cells (if they exist) is poorly known, in addition to the fact that they are expected to be Myf5-negative. Interestingly, Spiegelman and colleagues have recently identified a smooth muscle-like origin for at least a subset of beige/brite cells (35). In any case, it should be kept in mind that a difference in the developmental origin of adipocytes does not necessarily imply a functional difference. How important is the activity of the new beige/brite cells that appear as a consequence of browning of WAT relative to the activity of the classical BAT depots in rodents? First data indicated that cultured cells representative of the ‘classical’ brown and beige/brite lineages have similar rates of basal and uncoupled respiration (25). Moreover, a recent study that directly determined the uncoupling activity and metaboliteoxidizing capacity in mitochondria from beige/brite adipocytes compared with ‘classical’ brown adipocytes concluded that the two types of adipocytes showed very similar behavior (36). Only lower capacity of utilization of glycerol-3-phosphate in beige/ brite mitochondria relative to ‘classical’ brown mitochondria was reported. Theoretical calculations have suggested that the total amount of beige/brite cells cannot account for a large portion of energy expenditure relative to the capacity elicited by classical BAT depots (36). However, studies using different strains of mice characterized by distinct WAT browning capacities but a similar capacity to activate classical BAT have shown that browning capacity is highly correlated with protection against highfat-diet-induced obesity (37). In fact, a tendency toward lower thermogenic capacity was reported in mitochondria obtained from an obesity-prone mouse strain (C57Bl/6) compared with those from an obesity-resistant mouse strain (129Sv) (36). Further studies will be needed to reconcile the minor bioenergetic impact on the organism expected from browned WAT, determined on the basis of biochemical considerations, with the importance of the process evidenced by the genetic studies described above. Moreover, a potential endocrine role of BAT beyond its intrinsic thermogenic activity has been recently proposed (38); to date, there are no data indicating important differences in the bioactive factors (e.g. FGF21) released by beige/brite cells relative to ‘classical’ brown adipocytes (see below). Molecular controllers of differentiation of distinct adipocyte cell types Despite originating from different lineages, adipocytes undergo adipogenic differentiation processes that share common transcriptional cascades (39). Indeed, the nuclear receptor PPARγ (peroxisome proliferator-activated receptor-γ) is indispensable for the development of all types of adipose cells (40,41). The other master gene that determines adipogenic differentiation is C/EBPα (CCAAT/enhancer-binding protein-α). C/EBPα acts to maintain PPARγ expression, and both factors promote and maintain the differentiated state of adipocytes by co-operatively regulating the transcription of genes involved in processes such as lipid and glucose metabolism and insulin sensitivity (42). The absence of C/EBPα in mice prevents the development of all white, but not brown, adipose depots, indicating that a lack of C/EBPα can be compensated for in brown fat development, probably by C/EBPβ (43,44). In fact, C/EBPβ has been shown to play a key role in brown adipogenesis through direct interaction with the co-regulator PRDM16 (PR-domain containing protein-16) (45). PRDM16, together with PGC-1α (PPARγ-coactivator-1α), have been identified as the main regulators of the phenotype of both ‘classical’ brown adipocytes and beige/brite adipocytes. In particular, PGC-1α, which coactivates PPARγ and PPARα (46,47), is involved in the regulation of mitochondrial biogenesis, oxidative metabolism, and thermogenesis. In contrast, PRDM16, but not PGC-1α, has been shown specifically to confer brown fat cell identity (48,49). In fact, PRDM16 acts primarily through co-regulating C/EBPβ, PPARγ, PPARα, and PGC-1α to induce expression of brown fat-specific genes (16,45,49,50). MiR-133a/b and miR-155 are microRNA species that target PRDM16 and C/ EBPβ, respectively, thus having an inhibitory role on brown and beige/brite adipogenesis and function. In contrast, miR-196a appears to induce specifically beige/brite adipogenesis via inhibition of the homeobox protein HOXC8 (51). On the other hand, the co-regulator TLE3 competes with PRDM16 for binding to PPARγ, thus impairing brown adipocyte thermogenic gene expression and promoting a more white adipocyte gene expression signature (52). Recent studies of adipocyte-specific PRDM16knockout mice showed that ‘classical’ BAT remains functionally intact whereas there is a loss of the browning capacity (i.e. beige/ brite adipocyte function) in response to cold and β3-adrenergic stimulation. When exposed to a high-fat diet, those mice show an increase of subcutaneous WAT mass, but this WAT depot acquires physiological characteristics more related to visceral WAT (53). In contrast, selective ablation of PRDM16 in the brown (Myf5positive)—but not beige/brite (Myf5-negative)—adipose lineage results in altered ‘classical’ brown adipocyte identity and function in adult mice (54). Genetic markers of the beige/brite phenotype Several studies using differential gene expression profiling in cell cultures have identified specific genes whose expression may be used to distinguish beige/brite from brown adipocytes despite common expression of the thermogenic genes, such as UCP1 (29,55). For instance, differential high expression of the zinc finger transcription factor Zic-1 and LIM homeobox 8 protein (Lhx8) is commonly found in ‘classical’ brown adipocytes relative to beige/brite adipocytes in most studies (23,56). Expression of TNF receptor superfamily member 9 (CD137), T-box associated transcription factor (TBX1), transmembrane 26 (TMEM26), and short stature homeobox-2 (SHOX2) are considered indicative of beige/brite cell identity (25,56). The assessment of the expression of these genes in adipose tissue depots from mice confirmed partially that the differential gene expression signature occurs in vivo, (e.g. a high and distinctive expression of Zic-1 in ‘classical’ BAT depots but absence in beige/brite-prone WAT depots, or preferential expression of SHOX2 in inguinal WAT, a site highly sensitive to ‘browning’). Thus, it appears that marker gene expression profiles allows to distinguish a predominant ‘classical’ brown adipocyte phenotype in the BAT depots versus a predominant beige/brite phenotype in WAT depots in which browning had been induced (27). However, considering that several members of the gene panels that distinguish ‘classical’ BAT versus beige/ brite adipose tissue are homeobox genes or other genes involved in positional determination during mammalian development, it is unclear to what extent the expression of these marker genes in tissues is reflecting cell identity or just positional location of adipose cells across whole-body anatomy. In this sense, a recent Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. 4 R. Cereijo et al. approach aimed to identify a comprehensive molecular description of brown versus beige/brite gene expression using ribosomal profiling concluded that several candidate genes may in fact reflect much more the anatomical location in which adipocytes reside rather than intrinsic adipocyte type-specific identity (35). On the other hand, recent reports also claim that gene expression signature in adipose depots is highly dependent on the developmental stage even in the same anatomical depot (57). Finally, some authors have questioned the definition of beige/brite as an independent cell type with respect to classical brown adipocytes, and instead consider these cells should be named just brown adipocytes (58). In fact, white adipocytes from subcutaneous versus visceral depots are considered the same cell type, notwithstanding reported differences in developmental lineage, gene expression signature, and/or hormonal responsiveness (59–61). In the same sense, myocytes from different skeletal muscle fiber-types also constitute unique cell types (62). At this point, it may happen that, in fact, issues related to cell biology and those related to the preferred use of a given terminology to classify cells overlap. Signals that control beige/brite cell appearance and function Because prolonged thermogenic stimulation appears to be the main context in which the browning of WAT occurs in rodent models, the classically recognized regulatory axis of sympathetic activation and subsequent noradrenergic signaling is considered the main mechanism that elicits the appearance of beige/brite cells in WAT depots (63). However, a number of novel factors capable of inducing the browning process have been identified in recent years. Most of these factors also induce the activation of existing brown adipocytes and promote the recruitment of classical BAT anatomical depots. These new factors, which are capable of controlling the browning of WAT through mechanisms totally or partially independent of sympathetic activation, originate from distinct tissues. The discovery of one of these factors, irisin, is especially remarkable. Exercise has been reported to induce browning of WAT (64). Bostrom et al. (65) observed that overexpression in muscle of PGC-1α, a transcriptional co-regulator involved in muscular bioenergetics and metabolism, also caused browning of WAT. A search for the mechanisms responsible for this effect led to the identification of irisin, a bioactive protein released by skeletal muscle after cleavage of the membrane protein FNDC5 (fibronectin type III domain containing 5), and showed that this bioactive protein is capable of inducing the browning of WAT in response to muscle contraction. Another factor that promotes browning and activation of BAT is FGF21 (fibroblast growth factor-21) (66,67), a hormone released mainly by the liver in response to lipid availability (68). FGF21 is also released by white and brown adipocytes in rodents and may have both an endocrine and a paracrine action in promoting the browning and thermogenic activity of BAT (67,69). A number of other factors have been reported to promote browning and BAT activation in rodents, as recently reviewed (70). They include cardiac peptides (71), vitamin A derivatives (72) or even metabolites such as β-aminoisobutyric acid (73) and lactate (74). Human brown adipose tissue: ‘classical’ versus beige/brite How do the above concepts and findings, which have been developed mainly using cell culture models and rodents, apply to humans? Cell culture analysis to explore adipocyte lineage, as mentioned above for rodents, provided limited data for human brown-versus-beige/brite identity distinction on the basis of marker gene expression analysis. Table I summarizes the current findings relative to marker genes for the different subsets of adipocytes specifically in humans. Remarkable similarities in marker genes useful to distinguish adipocyte identity in adipose depots have been found relative to rodent studies and, also as in rodents, there is a wide range of biological roles for the corresponding gene products, most of them unrelated to the current knowledge of specific brown and beige/brite biological function. The discovery of active BAT in adult humans at the anatomical sites mentioned above has spurred interest in directly characterizing the features of this tissue through analysis of biopsies. In fact, in one of the original studies published in the New England Journal of Medicine (8), the authors reported high expression of UCP1 and other marker genes of BAT ⫹ beige/brite versus WAT molecular identity. The typical brown adipocyte morphology and expression of UCP1 were confirmed by an independent study in neck adipose tissue from adult humans (75). Recent studies have also attempted to determine whether adult BAT at upper trunk anatomical locations is ‘classical’ BAT or beige/brite BAT by direct analysis of fat at these anatomical sites. The first reports of these analyses yielded the surprising finding that BAT in the supraclavicular region of adult humans expressed the same marker genes representative of the beige/brite type previously identified in rodents and human cell culture studies (25). An additional study, performed using a set of samples of supraclavicular as well as visceral fat depots in neonates and children, also found indications of gene expression typical of beige/brite adipocytes, but no evidence for expression of markers of classical, developmentally programmed BAT (56). These findings raised the surprising possibility that all BAT in humans might be the beige/brite type. However, subsequent reports have challenged this concept. Lidell et al. (76) found that samples of BAT from the adult supraclavicular and periadrenal area expressed beige/brite markers genes, but an analysis of neonatal interscapular BAT, an anatomical site equivalent to the interscapular area in rodents, revealed differential expression of the classical BAT marker, ZIC1. In fact, on the basis of the expression of marker genes for classical BAT (i.e. ZIC1) and beige/brite BAT (i.e. CD137), we found that human fetuses at term simultaneously contain both types of brown adipocytes depending on the anatomical region: ‘classical’ BAT in the interscapular area and beige/brite in the omentum (77). At this point, the question becomes: does ‘classical’ BAT exist only in human fetuses and neonates, with all BAT in human adults being beige/brite? Even this formulation is challenged by recent findings. Cypess et al. (78) found that the deep fat layers in the necks of adults contain brown adipocytes (based on morphology and UCP1 expression) that show a pattern of gene expression (high ZIC1 expression) characteristic of classical BAT. In a separate study of neck adipose tissue from adults, the authors reached a similar conclusion, suggesting that human supraclavicular BAT might consist of both ‘classical’ brown and beige/brite adipocytes, also on the basis of marker gene expression (79). In summary, clarifying the identity of human brown adipocytes through direct studies of human biopsies and samples has proven to be a difficult task, first because of the limited availability and difficulty of obtaining such samples, and second because of inherent doubts about the suitability of directly applying profiles of ‘classical’ and beige/brite marker genes, derived mainly from rodent studies (and not totally agreed upon in the literature) to human BAT. Expression of the FGF21 gene highlights these difficulties. In rodents, FGF21 is substantially expressed in Brown and beige/brite adipogenesis in humans 5 Table I. Confirmed marker genes for different subsets of adipocytes in humans. Phenotype ‘Classical’ brown Gene symbol Biological function Zic family member 1 LHX8 LIM homeobox 8 miR206 MicroRNA 206 miR133b HOXC4 MicroRNA 133b Homeobox C4 HOXA1 UCP1 Homeobox A1 Uncoupling protein 1 DIO2 PRDM16 Deiodinase, iodothyronine, type II Peroxisome proliferator-activated receptor gamma, coactivator 1 alpha PR domain containing 16 ADRB3 FGF21 β3-adrenergic receptor Fibroblast growth factor 21 CIDEA Cell death-inducing DFFA-like effector A CITED1 TMEM26 SHOX2 Cbp/p300-interacting transactivator, with Glu/ Asp-rich carboxy-terminal domain, 1 T-box 1 Tumor necrosis factor receptor superfamily, member 9 Transmembrane protein 26 Short stature homeobox 2 Beige/brite ⫹ white HOXC8 Homeobox C8 White HOXC9 LEP Homeobox C9 Leptin ‘Classical’ brown ⫹ beige/brite PPARGC1A (PGC-1a) Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. Gene name ZIC1 Beige/brite TBX1 TNFRSF9 (CD137) both WAT and BAT (although FGF21 has been proposed to be preferentially expressed in beige/brite adipocytes in rodent cell culture studies) (25,56). However, in humans, FGF21 expression is negligible in WAT (both subcutaneous and visceral) (80), but is substantial in both classical and beige/brite fat depots from human neonates (77). BAT in pheochromocytoma patients: a human model of the browning of WAT Pheochromocytoma is a neuroendocrine tumor that secretes large amounts of catecholamines. More than 50 years ago, Feyrter identified the presence of adipocytes with the multilocular lipid droplet morphology typical of brown adipocytes in the fat depots close to pheochromocytoma tumors (81). Further research confirmed that these cells possessed the morphological (cytoplasm filled with mitochondria with numerous tightly packed cristae) and biochemical (mitochondrial guanosine diphosphate-sensitive, loose respiratory coupling) features of bona fide brown adipocytes (Figure 1) (82). Expression of the UCP1 gene and high amounts of UCP1 in mitochondria, an unequivocal sign of brown adipocyte identity, was also confirmed (83,84). In addition to UCP1, other BAT ⫹ beige/ brite marker genes (e.g. PRDM16, β3-adrenoreceptor) are also highly expressed in omental adipose tissue from pheochromocytoma patients (85). Moreover, recent PET scan studies have consistently reported hypermetabolism in the omental and Multifunctional C2H2-type zinc finger transcription factor Cysteine-rich double-zinc finger transcription factor Inhibition of muscular transcription factors myogenin and MyoD in brown adipocytes See miR206; regulation of PRDM16 expression Homeodomain-containing DNA-binding transcription factor See HOXC4 Uncoupling of oxidative metabolism from ATP production, allowing heat production Conversion of thyroxine (T4) to 3,3’,5triiodothyronine (T3) Transcriptional co-activator controlling the expression of key metabolism-related genes Transcriptional co-regulator, control of the brown phenotype transcriptional network Norepinephrine receptor Increase of glucose uptake and oxidation, induction of UCP1 Activator of apoptosis upon DNA fragmentation; lipolysis and thermogenesis regulator Transcriptional co-activator for estrogen receptors T-box-associated transcription factor Clonal expansion, survival, and development of T cells, induction of a Th1 program Unknown Homeodomain-containing DNA-binding transcription factor Homeodomain-containing DNA-binding protein See HOXC8 Regulation of body weight by inhibiting food intake and energy expenditure in adipocytes (Ref.) (55, 76, 78, 79) (55, 76, 78, 79) (27, 79) (79) (9) (9) (25, 55, 76, 78, 79) (76) (21, 76, 79, 108) (76, 79, 111) (76) (66, 77, 108) (79) (56, 79) (25, 55, 76, 79) (25) (25, 78, 79, 108) (76, 78, 112) (27, 79, 113) (27, 79, 108, 113) (78) mesenteric adipose tissue from pheochromocytoma patients, indicating the functionality of brown adipocytes that develop at those regions (86–89). According to current concepts, the brown adipocytes that appear in pheochromocytoma patients are, by definition, the consequence of a browning process because they appear at sites in which only WAT is present in control conditions and their appearance is dependent on a particular stimulus—in this case, continuous adrenergic stimulation caused by the tumor. Notably, under basal conditions, visceral adipose tissue, the anatomical site where BAT appears as a consequence of pheochromocytoma, is considered much less prone to browning than subcutaneous fat (a concept developed largely based on rodent studies), and remnant UCP1 expression levels in WAT from healthy adult humans is more prevalent in visceral than in subcutaneous fat (90). In any case, under this scenario, brown adipocytes that appeared in the visceral fat of pheochromocytoma patients would be expected to be beige/brite, but, to date, no extensive analysis of the molecular expression signature—‘classical’ versus beige/brite—has been reported in BAT from pheochromocytoma patients. How these adipocytes develop is the subject of a current controversy, fueled by results obtained in rodent models. Some authors suggest that precursor cells differentiate into a brown (beige/brite) phenotype, whereas others claim that the existence of morphologically intermediate forms between white and adipocytes—so-called paucilocular adipocytes, which express UCP1—supports the idea that a transdifferentiation process from Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. 6 R. Cereijo et al. Figure 1. Presence of beige/brite adipocytes in visceral adipose tissue from a patient with pheochromocytoma. Left: light microscopy, indicating the presence of multilocular beige/brite adipocytes among other unilocular white adipocytes. Right: electron microscopy of the area indicated in the left, high mitochondrial content characteristic of beige/brite adipocyte phenotype is shown, as well as poor mitochondria presence in the cytoplasmic rim of white adipocytes. white to beige/brite adipocytes takes place in adipose tissue from these patients (85). In any case, the appearance of brown adipocytes in patients with pheochromocytoma confirms the existence of browning potential in adipose tissues of adult humans. The availability of samples as a byproduct of surgical treatment procedures provides unique research opportunities that could be exploited to further elucidate the biological processes underlying adipose browning in humans. How is browning controlled in humans? Recent advances in our understanding of the neuro-hormonal control of the browning process in rodents (70,91,92) are beginning to be extended to humans, with the obvious limitations associated with human studies. As in rodents, a cold environment appears as a major inducer of human BAT activity (9,93). The pheochromocytoma-associated appearance of brown adipocytes in BAT depots is possibly the clearest evidence that in humans, as in rodents, chronic adrenergic stimulation is a major inducer of browning. Notably, the adrenergic receptor antagonist propanolol suppresses BAT activity in the neck and cervical areas of humans, as assessed by PET scans (94). This indicates that if most neck brown adipocytes are of the beige/brite type, as noted above, their activity is controlled by sympathetic-driven, adrenergic-mediated action. Some researchers have reported that the sympathomimetic ephedrine activates BAT in humans (95), whereas others were unable to replicate these findings (96), perhaps owing to differences in the dose administered. Do other, non-sympathetic-related, factors control BAT activity and the browning process in humans? Among the factors identified in previous rodent studies, irisin and FGF21 possibly deserve the most attention in humans. Investigations into the capacity of exercise to increase irisin levels in humans have led to conflicting results, with an initial report of higher irisin levels after endurance training (65) not being systematically confirmed by other independent studies (97–100). Whether exercise was even effective in promoting browning in human adipose tissue has been questioned (101). Some studies have indicated that irisin is unable to induce the expression of BAT-related marker genes in human white adipocytes (98), whereas others have reported that FNDC5 activates the thermogenic activity of neck (mostly beige/brite) adipocytes (102). It should be noted, however, that the reliability of current methods for measuring human irisin in blood has been a recent subject of debate, making it difficult to compare data from different laboratories (103,104). In summary, it appears that clear-cut evidence that irisin is able to induce the browning of human WAT is still lacking, and further research will be required (105,106). However, the recent finding that irisin is expressed and released by human WAT itself (107) and thus has a potential autocrine role in promoting browning may open new research directions that help to establish the actual function of irisin in human adipose tissue and its potential role in browning. With respect to FGF21, it should first be noted that, unlike the case in rodents, FGF21 in humans is very poorly expressed in WAT, but is highly expressed in BAT—both classical (interscapular BAT from neonates) and beige/brite (neck BAT from healthy humans, omental fat from pheochromocytoma patients) (77,108). There is evidence that human adipocytes are sensitive to FGF21 action, and treatment of human preadipocytes with FGF21 activates beige/brite genes involved in the browning program (102,108), suggesting that FGF21 may be an endocrine as well as an autocrine factor in controlling beige/brite appearance and activity. A recent report found a strong association between a rise in circulating FGF21 levels in response to cold and activation of BAT (mostly beige/brite) in the neck in humans, consistent with a role for FGF21 in promoting the activity of beige/brite cells (102). Conclusions and perspective Clarifying the cellular identity of distinct types of brown adipocytes may at a first glance appear to be somewhat of an academic exercise in the larger context of cell biology research. However, the current awareness of the potential value of promoting BAT activity in humans so as to enhance energy expenditure (thus protecting against obesity) and drain glucose and fat from the circulation (thus protecting against diabetes and hyperlipidemia) stresses the relevance of this line of research. Experiments in rodents suggest that the neurohormonal processes that control Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. Brown and beige/brite adipogenesis in humans 7 the activation and perhaps recruitment of existing BAT may not be identical to those that promote the enrichment of brown adipocytes in former WAT depots. Whereas some classical activators (e.g. β-adrenergic activation) and more recent activators (FGF21) appear to induce both the activity of existing BAT and the promotion of browning (66,67), others (e.g. irisin) (65) appear more selective for the browning process. However, the role of these factors in the control of BAT activity and browning of WAT specifically in humans has not yet been definitively established. Identification of cell targets in humans and determining their relative sensitivity to neurohormonal modulators is of utmost relevance to the identification of drug targets and development of pharmacological tools for future treatment of obesity and metabolic diseases. Moreover, although currently available data support common thermogenic roles for ‘classical’ brown and beige/brite adipocytes, their different gene expression profiles suggest that, beyond their current role as experimental tools, these cell types may potentially serve distinct, and as yet unidentified, physiological functions. Finally, new roles of BAT and/or beige/brite activation that could be used for metabolic therapy purposes are increasingly being proposed, ranging from the control of hyperlipidemia and hyperglycemia (109,110) to the alleviation of redox pressure (74). The fascinating story of adipose plasticity in humans and the prospects for pharmacological intervention are only just beginning. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Acknowledgements 21. This work was supported by MINECO (grant SAF2011-23636), Instituto de Salud Carlos III (grant PI11/00376), EU (FP7 project BETABAT, grant HEALTH-F2-2011-277713), and Generalitat de Catalunya (2009SGR-284). Thanks are given to A. Goday and J. M. Gallego-Escuredo for help with microscopy images from pheochromocytoma patients. 22. Declaration of interest: The authors report no conflicts of interest. 24. References 1. Lean MEJ, James PT. Brown adipose tissue in man. In: Trayhurn P, Nicholls DG, editors. Brown adipose tissue. Edward Arnold Ltd. London, UK; 1986. p. 339–65. 2. Rothwell NJ, Stock MJ. Regulation of energy balance. Annu Rev Nutr. 1981;1:235–56. 3. Cassard AM, Bouillaud F, Mattei MG, Hentz E, Raimbault S, Thomas M, et al. Human uncoupling protein gene: structure, comparison with rat gene, and assignment to the long arm of chromosome 4. J Cell Biochem. 1990;43:255–64. 4. Cunningham S, Leslie P, Hopwood D, Illingworth P, Jung RT, Nicholls DG, et al. The characterization and energetic potential of brown adipose tissue in man. Clin Sci (Lond). 1985;69:343–8. 5. Del Mar Gonzalez-Barroso M, Ricquier D, Cassard-Doulcier AM. The human uncoupling protein-1 gene (UCP1): present status and perspectives in obesity research. Obes Rev. 2000;1:61–72. 6. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–52. 7. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. 8. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. 9. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. 10. Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T, et al. Impact of UCP1 and β3AR gene polymorphisms on age-related 23. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. changes in brown adipose tissue and adiposity in humans. Int J Obes (Lond). 2013;37:993–8. van Marken Lichtenbelt W. Brown adipose tissue and the regulation of nonshivering thermogenesis. Curr Opin Clin Nutr Metab Care. 2012;15:547–52. Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, et al. Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging. 2014;41:776–91. Moulin K, Truel N, André M, Arnauld E, Nibbelink M, Cousin B, et al. Emergence during development of the white-adipocyte cell phenotype is independent of the brown-adipocyte cell phenotype. Biochem J. 2001;356:659–64. Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, et al. The generation of adipocytes by the neural crest. Development. 2007;134:2283–92. Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta. 2014;1842:340–51. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011; 108:143–8. Sanchez-Gurmaches J, Hung CM, Sparks C, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16:348–62. Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by b3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15;480–91. Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–8. Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–25. Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, et al. Brown-fat paucity due to impaired -BMP signalling induces compensatory browning of white fat. Nature. 2013;495,379–83. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–14. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76. Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. Waldén TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012; 302:E19–31. Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: whitebrown adipocyte plasticity and metabolic inflammation. Obes Rev. 2012;13(Suppl 2):83–96. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–81. Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298: E1244–53. Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–29. Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013; 15:659–67. Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–44. Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. 8 R. Cereijo et al. 35. Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–20. 36. Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–203. 37. Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–20. 38. Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab. 2013;305:E567–72. 39. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. 40. Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. 41. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPARgamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–17. 42. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, et al. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–8. 43. Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, et al. C/EBPalpha is required for differentiation of white, but not brown, adipose tissue. Proc Natl Acad Sci U S A. 2001; 98:12532–7. 44. Carmona MC, Iglesias R, Obregón MJ, Darlington GJ, Villarroya F, Giralt M. Mitochondrial biogenesis and thyroid status maturation in brown fat require CCAAT/enhancer-binding protein alpha. J Biol Chem. 2002;277:21489–98. 45. Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–8. 46. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. 47. Barbera MJ, Schluter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. 2001;276:1486–93. 48. Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–41. 49. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. 50. Hondares E, Rosell M, Díaz-Delfín J, Olmos Y, Monsalve M, Iglesias R, et al. Peroxisome proliferator activated receptor-alpha (PPARα) induces PPARγ- coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J Biol Chem. 2011;286:43112–22. 51. Trajkovski M, Lodish H. MicroRNA networks regulate development of brown adipocytes. Trends Endocrinol Metab. 2013;24:442–50. 52. Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab. 2013;17:423–35. 53. Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014; 156:304–16. 54. Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, et al. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19:593–604. 55. Scheele C, Larsen TJ, Nielsen S. Novel nuances of human brown fat. Adipocyte. 2014;3:54–7. 56. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452 57. Chu DT, Malinowska E, Gawronska-Kozak B, Kozak LP. Expression of adipocyte biomarkers in a primary cell culture model reflects pre-weaning adipobiology. J Biol Chem. 2014;289:18478–88. 58. Rosenwald M, Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3:4–9. 59. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. 60. Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17:644–56. 61. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. 62. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–531. 63. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103: 931–42. 64. Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1115–25. 65. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1á-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. 66. Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–12. 67. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81. 68. Adams AC, Kharitonenkov A. FGF21: the center of a transcriptional nexus in metabolic regulation. Curr Diabetes Rev. 2012;8:285–93. 69. Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–90. 70. Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–43. 71. Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–36. 72. Villarroya F, Iglesias R, Giralt M. Retinoids and retinoid receptors in the control of energy balance: novel pharmacological strategies in obesity and diabetes. Curr Med Chem. 2004;11:795–805. 73. Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. 74. Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014 May 1. [Epub ahead of print]. 75. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–20. 76. Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–4. 77. Hondares E, Gallego-Escuredo JM, Flachs P, Frontini A, Cereijo R, Goday A, et al. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism. 2014;63:312–17. 78. Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–9. 79. Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. 80. Gallego-Escuredo JM, Gómez-Ambrosi J, Catalan V, Domingo P, Giralt M, Frühbeck G, et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes (Lond). 2014 May 12. [Epub ahead of print]. 81. Feyrter F. On the degeneration of brown fatty tissue in pheochromocytoma. Wien Med Wochenschr. 1961;111:648–9. 82. Ricquier D, Nechad M, Mory G. Ultrastructural and biochemical characterization of human brown adipose tissue in pheochromocytoma. J Clin Endocrinol Metab. 1982;54:803–7. 83. Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes (Lond). 1986;10:219–27. Ann Med Downloaded from informahealthcare.com by Universitat de Barcelona on 10/27/14 For personal use only. Brown and beige/brite adipogenesis in humans 9 84. Bouillaud F, Villarroya F, Hentz E, Raimbault S, Cassard AM, Ricquier D. Detection of brown adipose tissue uncoupling protein mRNA in adult patients by a human genomic probe. Clin Sci (Lond). 1988;75:21–7. 85. Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta. 2013;1831:950–9. 86. Hadi M, Chen CC, Whatley M, Pacak K, Carrasquillo JA. Brown fat imaging with (18)F-6-fluorodopamine PET/CT, (18)F-FDG PET/CT, and (123)I-MIBG SPECT: a study of patients being evaluated for pheochromocytoma. J Nucl Med. 2007;48:1077–83. 87. Wang Q, Zhang M, Ning G, Gu W, Su T, Xu M, et al. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS One. 2011;6:e21006. 88. Cheng W, Zhu Z, Jin X, Chen L, Zhuang H, Li F. Intense FDG activity in the brown adipose tissue in omental and mesenteric regions in a patient with malignant pheochromocytoma. Clin Nucl Med. 2012;37:514–15. 89. Dong A, Wang Y, Lu J, Zuo C. Hypermetabolic mesenteric brown adipose tissue on dual-time point FDG PET/CT in a patient with benign retroperitoneal pheochromocytoma. Clin Nucl Med. 2014; 39:e229–32. 90. Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res. 1997; 38:2125–33. 91. Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–85. 92. López M, Alvarez CV, Nogueiras R, Diéguez C. Energy balance regulation by thyroid hormones at central level. Trends Mol Med. 2013; 19:418–27. 93. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. 94. Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging. 2007;34:1018–22. 95. Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–55. 96. Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–5. 97. Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:E9–10. 98. Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, et al. Evidence against a beneficial effect of irisin in humans. PLoS One. 2013;8:e73680. 99. Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S, et al. Irisin and exercise training in humans - results from a randomized controlled training trial. BMC Med. 2013;11:235. 100. Pekkala S, Wiklund PK, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591:5393–400. 101. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–49. 102. Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9. 103. Erickson HP. Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte. 2013;2:289–93. 104. Sanchis-Gomar F, Alis R, Pareja-Galeano H, Romagnoli M, Perez-Quilis C. Inconsistency in circulating irisin levels: what is really happening? Horm Metab Res. 2014;46:591–6. 105. Irving BA, Still CD, Argyropoulos G. Does IRISIN have a BRITE future as a therapeutic agent in humans? Curr Obes Rep. 2014;3: 235–41. 106. Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol. 2014;222:R25–38. 107. Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belén Crujeiras A, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. 108. Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond). 2014;38:170–6. 109. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. 110. Peirce V, Vidal-Puig A. Regulation of glucose homoeostasis by brown adipose tissue. Lancet Diabetes Endocrinol. 2013;1:353–60. 111. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. 112. Lee KY, Yamamoto Y, Boucher J, Winnay JN, Gesta S, Cobb J, et al. Shox2 is a molecular determinant of depot-specific adipocyte function. Proc Natl Acad Sci U S A. 2013;110:11409–14. 113. Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–6.