07 Practice Problems

Unit 7 Practice Problems
1) For each of the following compounds indicate which intermolecular force is most important:
FCN ____________________________
HCN ___________________________
C
2
H
6
_ London __________________________
CF
2
H
2
_ dipole __________________________
2) Explain why ethyl alcohol (C
2
H
5
OH) has a higher boiling point (78.4
0
C) than methyl alcohol
(CH
3
OH; 64.7
0
C).
H H H
| | |
Methyl alcohol
H ‒ C ‒ O ‒ H
|
H
Ethyl alcohol
H ‒ C ‒ C ‒ O ‒ H
| |
H H
Ethyl alcohol and methyl alcohol both have London, Dipole, and H-bond forces. Since ethyl alcohol has more electrons, its London forces are stronger so its boiling point is higher.
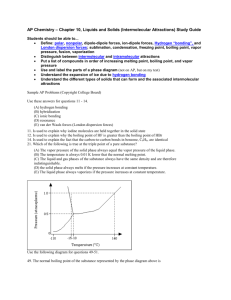
3) The vapor pressures of four substances at various temperatures are shown in the graph. Use this graph to answer the questions below. a. What is the normal boiling temperature of chloroform and ethanoic acid, respectively?
Look at the vapor pressure when it matches the standard barometric pressure
(101.3KPa), Chloroform is 61ºC, while ethanoic acid is 121ºC b. At 60 KPa, water would boil at what temperature?
87ºC c. If atmospheric pressure was 80 KPa, which substances could boil at 60 o C?
Chloroform could d. Which of these substances has the highest intermolecular attractions?
Ethanoic acid
4) Calculate the heat of vaporization of carbon disulfide which has a vapor pressure of 400.0 mmHg at 28.0
˚ C and a normal boiling point of 46.5
˚ C. ln
400.0
760.0
H vap
1
1
8.31 28 273.15 465 273.15
H vap
27700
J mol
5) Rank the following by from lowest to highest anticipated boiling point: C
2
H
4
, CH
4
, H
3
COCH
3
.
Lowest B.P.: CH
4
< C
2
H
4
< H
3
COCH
3
:Highest B.P.
H H H
| | |
H ‒ C ‒ H
|
H
|
H
|
H ‒ C
|
‒ O ‒ C
|
‒ H
H H ‒ C = C ‒ H H H
All three have London forces, but CH
4
has the weakest ones because it has fewer total electrons than either of the other molecules. This causes CH
4
to have the lowest boiling point.
H
3
COCH
3
has a bent structure around the oxygen atom; therefore, this molecule is polar and carries dipole forces in addition to its London forces. This means that the stronger attractions within this molecule will cause the highest boiling point.
6) Motor oil largely consists of molecules that consist of long chains of carbon atoms with hydrogen atoms attached to them. Using your knowledge of intermolecular forces, why wouldn’t it be better to use a compound like glycerol. The formula of glycerol is CHOH(CH
2
OH)
2
.
Glycerol would have too many hydrogen bonds making it highly viscous
(too sticky, meaning too much friction)
7) Arrange the following in order of increasing boiling point:
Ar, He, Ne, Xe
Lowest B.P.: He < Ne < Ar < Xe :Highest B.P.
All of these noble gases experience London forces, so the difference in their strengths is dependent on the number of electrons within each atom. The atom with the least number of electrons, He, has the weakest attractions. This means that He also has the lowest boiling point, meaning it is easiest to boil. Xe has the most electrons so it carries the strongest Long forces and the highest boiling point.
8) A pure solid substance is heated as indicated in the diagram. Use the diagram answer questions the questions below. a. On which portion(s) of the graph is only
a liquid present? C b. On which portion(s) of the graph is a liquid (and maybe other phases of matter) present?
B,C,D c. On which portion(s) of the graph is only
a gas present? E d. Which section of the graph indicates the boiling point? D
e. Which section of the graph indicates the freezing point? B f. On which portion(s) of the graph would you use the formula: q = mc Δ T, to calculate the energy change? A,C,E g. On which portion(s) of the graph would you use the formula, number of moles Δ H vap
, to calculate the energy change? D
9) Carbon tetrachloride was once widely used in the dry cleaning industry. Its use was discouraged, however, after it was determined to be carcinogenic. It has a vapor pressure of 9.708 mmHg at
‒ 20.0 °C and 90.00 mmHg at 20.0 °C. Use the Clausius-Clapeyron equation to estimate the a. heat of vaporization
H
8.31
vap
J
1
1
20.0 273.15 20.0 273.15
H vap
34300 J mol b. temperature at which the vapor pressure is 0.50 atm.
0.50
atm
760 mmHg
380 mmHg
1 atm
34300
8.31
J
J mol
1
1
T
T
327
K
10) Mercury is used extensively in studying the effect of pressure on the volume of gases. It is fairly inert at the surface, and most gases are insoluble in it. Should we worry about the contribution of the vapor pressure of mercury to the pressure of gases above it? Use the following data to determine the vapor pressure of mercury at 0 °C and 100 °C: º ∆ H vap
of Hg = 59.4 kJ/mol; normal boiling point
= 357 °C.
ln
59400
8.31
ln
ln
8.19
14.825
J mol
J
vp
2.77 10 4 mmHg
1
1
357 273.15 0 273.15
ln
59400
8.31
J
J mol
vp
0.306
mmHg
1
1
357 273.15 100 273.15
11) Iodine has a triple point at 114 °C, 90 mmHg. Its critical temperature is 535 °C. The density of the solid is 4.93 g/cm 3 , while that of the liquid is 4.00 g/cm 3 . Sketch a phase diagram for iodine, and use it to fill in the blanks below, using either “liquid” or “solid.” a. Iodine vapor at 80 mmHg condenses to the solid when cooled sufficiently. b. Iodine vapor at 125 °C condensed to the solid when enough pressure is applied. c. Iodine vapor at 700 mmHg condenses to the solid when cooled above the triple point temperature.
12) A pure substance A has a liquid vapor pressure of 320 mmHg at 125 °C, 800 mmHg at 150 °C, and
60 mmHg at the triple point, 85 °C. The melting point of A decreases slightly as pressure increases. a. Sketch a phase diagram for A. b. From the phase diagram, estimate the normal boiling point. Approximately 145ºC at 760 mmHg c. What changes occur when at a constant pressure of 320 mmHg, the temperature drops from 150 to 100 °C? Liquid A would technically be a gas at 150ºC and 320 mmHg. As the temperature cools, it would become a liquid and then a solid.
13) Explain in terms of intermolecular forces why a. ICl has a higher melting point than Br
2
.
ICl has dipole and London forces, while Br
2
only has London forces. This means that ICl has stronger attractions, which make it more difficult to melt, causing a higher melting point. b. C
2
H
6
has a higher boiling point than CH
Both C
2
H
6
and CH
4
4
.
only have London forces, but C2H6 has more total number of electrons.
This increases the attractions found between molecules of C
2
H
6
and requires more energy to boil.
c. H
2
O
2
has a higher boiling point than C
2
H
6
.
H d. C
2
O
2
H
H
2
5
has dipole and London forces, while C
2
H
6
only has London forces. This means that H
2
O
2 has stronger attractions, which make it more difficult to boil, causing a higher boiling point.
C
2
5
OH has a lower boiling point than NaF.
OH is molecular with London, Dipole, and H-bonding attractions, while NaF is ionic with electrostatic attractions. Since NaF would have to break intramolecular forces, which are stronger than any intermolecular forces of C
2
H
5
OH, its boiling point would be higher.
14) What are the strongest intermolecular forces that must be overcome to a. boil silicon hydride (SiH
4
)? forces b. melt iodine (I
2
)? forces c. dissolve chlorine in carbon tetrachloride?
Cl
2
would be able to form London forces with CCl
4 d. vaporize calcium chloride?
CaCl
2 (s)
→ Ca 2+
(g)
+ 2 Cl ‒
(g)
; electrostatic attractions/ionic bonds
15) Of all the general categories of solids, which one(s) a. are generally low boiling? molecular b. are ductile and malleable? metallic c. are generally nonvolatile? metallic, ionic, network covalent, and amorphous
16) Classify each of the following species as molecular, network covalent, ionic, or metallic: a. K metallic b. CaCO
3 ionic c. C
8
H
18 molecular
(diamond) network covalent e. HCl molecular-ionic