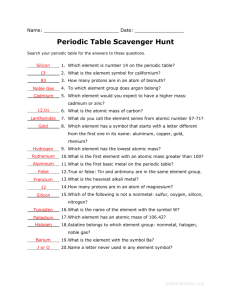
NOTE: ALL OF THE ATOMS ARE NEUTRAL 32 42 222 86 33 27 30
advertisement
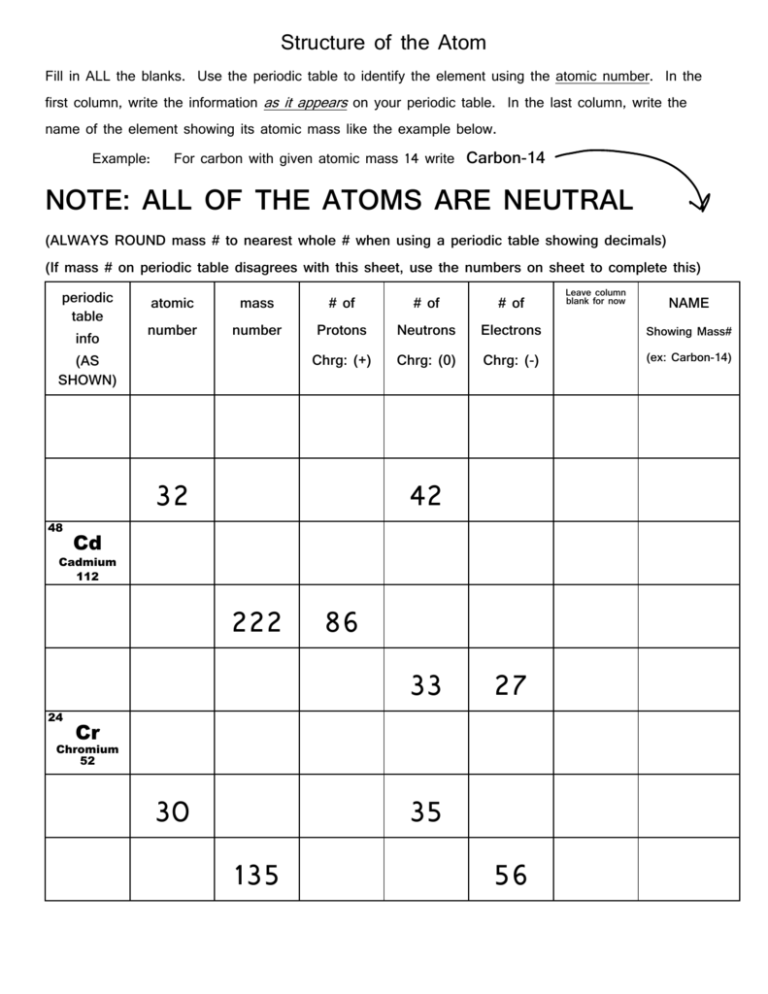
Structure of the Atom Fill in ALL the blanks. Use the periodic table to identify the element using the atomic number. In the first column, write the information as it appears on your periodic table. In the last column, write the name of the element showing its atomic mass like the example below. Example: For carbon with given atomic mass 14 write Carbon- NOTE: ALL OF THE ATOMS ARE NEUTRAL (ALWAYS ROUND mass # to nearest whole # when using a periodic table showing decimals) (If mass # on periodic table disagrees with this sheet, use the numbers on sheet to complete this) periodic table info (AS SHOWN) Leave column blank for now atomic mass # of # of # of number number Protons Neutrons Electrons Showing Mass# Chrg: (+) Chrg: (0) Chrg: (-) (ex: Carbon-) 32 42 48 Cd Cadmium 112 222 86 33 27 24 Cr Chromium 52 30 35 135 56 NAME