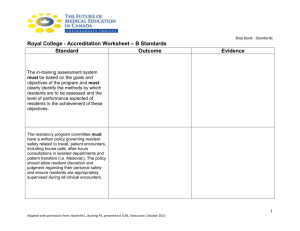
Part I Handouts - American Medical Directors Association
advertisement

CORE CURRICULUM ON MEDICAL DIRECTION IN LONG TERM CARE July 21-27, 2012 Baltimore, MD Core Curriculum on Medical Direction in Long Term Care July 21-27, 2012 Baltimore, MD SCHEDULE AT A GLANCE: PART I TOPIC DATE / TIME Saturday, July 21, 2012 AGENDA ITEM FACULTY LOCATION Grand Ballroom Foyer 3:30 PM - 4:30 PM REGISTRATION Topic: 01 4:30 PM - 5:00 PM Course Introduction Burl Grand Ballroom Topic: 02 5:00 PM - 6:00 PM Overview of Long Term Care Lecture Winn Grand Ballroom 6:00 PM – 6:30 PM Small Group Breakout All Faculty Breakouts 6:30 PM – 7:30 PM MEET AND GREET Grand Ballroom Foyer 7:00 AM - 7:30 AM BREAKFAST Grand Ballroom Foyer 7:30 AM - 9:45 AM Regulatory Environment Lecture 9:45 AM - 10:00 AM BREAK Sunday, July 22, 2012 Topic: 03 Topic: 03 10:00 AM - 11:00 AM Topic: 04 11:00 AM - 12:00 PM 12:00 PM - 1:30 PM Regulatory Environment Breakouts Medical Information Management Lecture Sponsored Lunch Symposium: Practical Considerations in Stroke Risk Reduction in Non-Valvular Atrial Fibrillation Leible/Baker Grand Ballroom Foyer All Faculty Breakouts Kaplan/Baker Grand Ballroom Sponsored University Ballroom All Faculty Breakouts Kaplan/Baker Grand Ballroom Bluestein Grand Ballroom Provided by Boehringer Ingelheim Topic: 04 1:30 PM - 2:15 PM Topic: 04 2:15 PM - 2:30 PM Topic: 05 2:30 PM - 3:30 PM 3:30 PM - 3:45 PM Speaker: Steven N. Singh, MD Medical Information Management Breakouts Medical Information Management (Wrap-Up) Employee Health and Safety Lecture BREAK Grand Ballroom Grand Ballroom Foyer 1 TOPIC DATE / TIME AGENDA ITEM FACULTY LOCATION Topic: 06 3:45 PM - 5:15 PM Infection Control Lecture Kaplan/ Brechtelsbauer Grand Ballroom Monday, July 23, 2012 Grand Ballroom Foyer 7:15 AM - 7:45 AM BREAKFAST Topic: 06 7:45 AM - 8:45 AM Infection Control Breakouts All Faculty Breakouts Topic: 07 8:45 AM - 10:15 AM Residents Rights Lecture Brubaker/Bluestein Grand Ballroom 10:15 AM - 10:30 AM BREAK Topic: 08 10:30 AM - 11:45 AM Financial Issues Lecture Brubaker Grand Ballroom Topic: 08 11:45 AM – 12:00 AM Financial Issues: Coding Lecture Baker Grand Ballroom 12:00 AM - 1:00 PM Lunch: In-The-Trenches Topic: 08 1:00 PM - 3:00 PM 3:00 PM - 3:30 PM Financial Issues: Coding (Continued) Focus Session and Evaluation of Part I Grand Ballroom Foyer University Ballroom Baker Grand Ballroom Burl Grand Ballroom 2 Core Curriculum on Medical Direction in Long Term Care July 21-27 Baltimore, MD SCHEDULE AT A GLANCE: PART II TOPIC DATE / TIME Tuesday, July 24, 2012 Topic: 09 Topic: 10 Topic: 10 FACULTY LOCATION Grand Ballroom Foyer 7:00 AM - 7:30 AM BREAKFAST 7:30 AM - 7:35 AM Introduction and Overview of Part II 7:35 AM – 7:45 AM CMD Presentation 7:45 AM - 7:55 AM Personality Profiles Worksheet Burl Grand Ballroom 7:55 AM - 9:50 AM Introduction to Medical Care Delivery Systems Lecture Brechtelsbauer Grand Ballroom 9:50 AM - 10:05 AM BREAK 10:05 AM - 11:30 AM Essential Health Information & Tools Lecture 11:30 AM - 12:30 PM LUNCH 12:30 PM - 1:40 PM Essential Health Information & Tools Breakouts 1:40 PM - 1:55 PM BREAK Topic: 10 1:55 PM - 3:35 PM Topic: 11 3:35 PM - 5:10 PM Topic: 12 AGENDA ITEM Essential Health Information & Tools Lecture (Continued) Medical Director’s Contract Lecture Burl Grand Ballroom Grand Ballroom Grand Ballroom Foyer Leible/Baker Grand Ballroom University Ballroom All Faculty Breakouts Grand Ballroom Foyer Leible/Baker Grand Ballroom Burl Grand Ballroom Grand Ballroom Foyer 5:10 PM - 5:30 PM BREAK 5:30 PM - 6:30 PM Personality Profiles Lecture Burl Grand Ballroom Wednesday, July 25, 2012 Topic: 13 7:45 AM - 9:00 AM Influencing Empl. Behaviors Lecture w/ BREAKFAST Bluestein Grand Ballroom Topic: 14 9:00 AM - 10:00 AM Medical Staff Oversight Lecture Kaplan Grand Ballroom 10:00 AM - 10:15 AM BREAK Topic: 14 10:15 AM - 11:00 AM Topic: 14 11:00 AM - 11:30 AM Medical Staff Oversight Breakouts Medical Staff Oversight Lecture (Wrap-Up) Grand Ballroom Foyer All Faculty Breakouts Kaplan Grand Ballroom 3 TOPIC DATE / TIME AGENDA ITEM FACULTY LOCATION 11:30 AM - 1:00 PM Sponsored Lunch Symposium: MDS 3.0 and Management of Moderate to Severe Alzheimer's Disease in LTC Sponsored University Ballroom Provided by Forest Pharmaceuticals, Inc. Speaker: Roger J. Cadieux, MD Topic: 15 1:00 PM – 2:15 PM Biomedical Ethics Lecture Winn Grand Ballroom Topic: 15 2:15 PM – 3:00 PM Biomedical Ethics Breakouts All Faculty Breakouts 3:00 PM – 3:15 PM BREAK 3:15 PM – 5:15 PM Working with Families Lecture Topic: 16 Grand Ballroom Foyer Brechtelsbauer Grand Ballroom Thursday, July 26, 2012 Grand Ballroom Foyer 7:30 AM – 8:00 AM BREAKFAST 8:00 AM - 8:05 AM Opening Remarks Burl Grand Ballroom 8:05 AM - 9:05 AM Quality Management Lecture Bluestein/Leible Grand Ballroom 9:05 AM - 9:20 AM BREAK Topic: 17 9:20 AM - 10:00 PM Quality Management Cont. Bluestein/Leible Grand Ballroom Topic: 17 10:00 AM – 12:00 PM Quality Management Breakouts All Faculty Breakouts 12:00 PM – 1:00 PM LUNCH 1:00 PM – 2:30 PM Risk Management Lecture 2:30 PM - 2:45 PM BREAK Topic: 19 2:45 AM – 3:45 PM Systems Theory Lecture Brechtelsbauer Grand Ballroom Topic: 19 3:45 PM - 4:45 PM Systems Theory Breakouts All Faculty Breakouts Topic: 19 4:45 PM - 5:15 PM Systems Theory Lecture (Wrap-Up) Brechtelsbauer Grand Ballroom 5:15 PM - 5:30 PM Focus Session and Evaluation Burl Grand Ballroom 5:30 PM - 6:00 PM Workshop on Action Plan Burl Grand Ballroom Topic: 17 Topic: 18 Grand Ballroom Foyer University Ballroom Winn/Kaplan Grand Ballroom Grand Ballroom Foyer Friday, July 27, 2012 Grand Ballroom Foyer 6:30 AM - 7:00 AM BREAKFAST Topic: 20 7:00 AM - 7:45 AM Governance Lecture Brubaker Grand Ballroom Topic: 21 7:45 AM - 8:30 AM Committees Lecture Brubaker Grand Ballroom 8:30 AM – 8:45 AM BREAK Topic: 21 8:45 AM – 9:15 AM Committees Breakout All Faculty Breakouts Topic: 22 9:15 AM - 11:15 AM Leadership in the Organization Lecture Burl Grand Ballroom Grand Ballroom Foyer 4 TOPIC DATE / TIME AGENDA ITEM FACULTY LOCATION 11:15 AM – 11:30 AM Closing Remarks Burl Grand Ballroom 5 Core Curriculum on Medical Direction in Long Term Care DAILY REMINDERS and INFORMATION Welcome to the AMDA Core Curriculum on Medical Direction in Long Term Care! We are very excited about the week ahead since we know the course will offer you opportunities for interaction with one another and faculty, as well as the chance to provide feedback through the audience response system (the keypads you see on your desks) and several evaluations – allowing us to check in with you to learn your thoughts as we go along. The information you provide us is vital to us as we attempt to tailor the Course onsite to your needs and wishes as a group, and later, to shape future courses to better meet the overall needs of students in medical direction. We know you’ll find our work this week intensive, challenging and rewarding. MATERIALS As far as materials go, you have each received a bag with several items. The bag includes a flash drive containing the course materials, slide sets, answer keys and JAMDA reference information. You have also been provided with an attendee folder including the course agenda, CME tracking form, commitment to change form, MDS 3.0 booklet, two blank Individualized Action Plan forms and course evaluation. NOTE: If you will only be joining us for Part I you will not have the course materials for Part II. We hope you enjoy these resources, a small sampling of the products and tools AMDA has to offer. AMDA staff will have a display set up later in the week with products available for sale. We encourage you to take a look. BREAKOUTS For several modules, we’ll be working in small groups in breakout sessions. For these sessions you have been given a participant workbook, which is located on your flash drive. Please bring the flash drive with you to the breakout session. FORMING GROUPS If you have not already done so, please complete the Pre-Course Assessment Form and return to the AMDA staff ASAP. We will use this information to place you in the appropriate small group assignment. Group assignments are posted on a board near the registration desk. EVALUATIONS AND ASSESSMENTS One goal of this course is to measure learning and changes in attitude at various points throughout the week. In addition to the pre-course assessment, we ask that you complete the evaluation form we’ve provided. At the course conclusion, you will also be asked to complete 2 Individualized Action Plan (IAP) forms. In approximately 6-months you will receive a Post-Course Assessment to complete and return. Please do not forget to include your name on all forms. We need to be able to match pre and post assessments to collect data. Your names will only be used for culling information and will not be noted or referenced in any other way. 6 CME TRACKING FORM The CME Tracking Form located in your Attendee Folder is a 2-part form. Do not lose it. This is your record and ours of your participation in this conference. Remember that you must attend both morning and afternoon sessions to make the most of your educational experience. Please keep one copy to serve as YOUR certificate of attendance and drop off the other at the registration desk at the end of the week. A separate certificate will NOT be mailed to you after the conference. SIGNING IN Please be sure to sign in each morning and afternoon at the registration desk. We will use the sign-in sheets to verify your attendance. You need to sign in twice a day. BATHROOMS Restrooms are located outside of the General Session room and will be directly on your right. PHONES We ask that you limit distractions by turning off your cell phones or turning them to vibrate. LUNCH If you requested a special meal, please be sure to bring your meal ticket and give it to your server to ensure the appropriate meal. Please return the following forms to AMDA staff by the end of the week: - Evaluation Form - The CME Tracking Form - Pre-course Assessment Form (If you have not previously filled it out online) - White Copy of the Two IAP Forms - White copy of the Commitment to Change Form Thank you and we look forward to learning your thoughts on your evaluation forms and to keeping in touch with you as AMDA follows up with your progress on your Individual Action Plans. 7 Core Curriculum on Medical Direction in Long Term Care WHAT HAPPENS AT THE END OF THE CORE? 1. At the end of Part II, Core attendees complete 2 Individualized Action Plans (IAP) based on the Functions and Tasks that have been covered throughout the Core. 2. These IAPs can only be done after the full completion of the entire Core Curriculum (parts I and II) as the knowledge from both parts is needed to fully implement any plan of action. 3. Development of the IAP is considered to be part of the course Part I and Part II and is required to be completed in order to obtain the 46 credit hours of CME/CMD for the course. 4. Return a copy of your IAPs to staff and keep a copy as your guide and reminder. 5. Additionally sign your ‘Commitment to Change’ form and return one copy to AMDA WHAT HAPPENS AFTERTHE CORE? 1. Once back in your practice setting, take steps to implement your action plans. 2. Over the following 6 months, you will receive a reminder(s) from faculty to be working on your action plan 3. At 6 months post-Core, AMDA staff will send you a Post-Course Assessment to complete and return. 4. In order to obtain all of the 20 additional Performance Improvement (PI) credit hours of CME/CMD, an attendee must complete the course in its’ entirety. Full completion of the course includes: Pre-Course Assessment Part I and Part II of the course Development of the IAP Implementation of the IAP (successfully or not, with reasons) Post-Course Assessment. The 20 additional credits is a separate activity from the 46 hours awarded for full participation at Part I and Part II of the Core Curriculum. The 20-hour activity is a performance improvement activity which allows learners to selfassess and implement improvement to their practice over time. There is no partial credit for the PI portion of the course. 8 CORE CURRICULUM ON MEDICAL DIRECTION Learning Objectives Part I Part I Overall Describe the framework and expected outcome of the course work. Explain the concept and levels of care in the continuum of long term care. Discuss the effects of influencing factors and emerging trends on the continuum of care. Identify regulatory requirements and delineate how the medical director can assist the facility in compliance. Assess the survey process and the medical director’s role in the process. Recognize the components and functions of a comprehensive medical record in long term care and employ processes to ensure the integrity and usefulness of the medical record. Develop and recommend ways to monitor infectious disease and improve infection control within the facility. Critique components and processes that provide adequate employee health and safety programs. Integrate awareness of residents’ rights into the differing scopes of practice of medical director and attending physician within an ethical framework. Define the medical director’s functions and tasks relative to financial issues in long term care facilities. Topic Topic Objectives 01 Course 1. Delineate the content, format and rationale of the Core Curriculum. Introduction 2. Define roles, functions and tasks as they apply to medical direction. 3. Describe the behavioral expectations for the participants after the course, including the development of a personalized action plan. 4. Share data about perceptions of participants’ current behavior. 02 Overview of Long Term Care 1. Discuss the history and evolution of systems of long term care. 2. Understand the concept of the continuum of care and identify key organizations that provide that care. 3. Identify the levels of care provided and the differences between delivery sites. 4. Be able to match the needs of long term patients with the appropriate level of care. 5. Describe the influence of other factors in the long-term care environment. 6. Understand the effect of emerging trends and patterns on the roles and responsibilities of long term care organizations. 03 Regulatory Environment 1. List the long term care regulatory agencies and describe their process of developing and enforcing regulations. 2. Describe the survey process, the types of surveys, and responses to deficiencies. 3. Delineate the ways in which the medical director may assist the facility in complying with local, state and federal regulations. 4. Define medical director’s role in a survey visit. 5. Describe the role of the medical director and the associated investigative protocol. 6. Describe the special emphasis and regulations regarding medication use in long-term care. 04 Medical Information Management 1. Recognize the components and describe the functions of a comprehensive medical record in long term care. 2. Describe the tasks of the medical director that help ensure the integrity and clinical usefulness of the medical record. 3. Describe and use a process to critique and improve the usefulness of the medical record. 4. Describe legal and regulatory forces that may impact clinical data. 5. Recognize existing computer technologies designed to facilitate medical record keeping and promote effective use of facility-wide data. 6. Recognize the differences in record-keeping between nursing facility and non-nursing home settings. 9 CORE CURRICULUM ON MEDICAL DIRECTION 05 Employee Health & Safety Learning Objectives 1. Describe components and processes of an effective employee health program. 2. List important (common and uncommon, but serious) illnesses and injuries seen in the LTC setting. 3. Assess the adequacy of the employee health and safety program at the participant’s facility. 4. Define the medical director’s tasks that contribute to a successful facility employee health program, including workman’s compensation. 5. Manage the potential ethical and legal conflicts resulting from establishing a physician-patient relationship with an employee while having a fiduciary relationship with the facility. 06 Infection Control 1. Develop or make recommendations for improving the infection control program in the participant’s facility. 2. Help control and prevent important (common, or uncommon but serious or emerging) infectious illnesses dealt with in the LTC continuum, including particularly nosocomial infections. 3. State the regulatory basis for an infection control program. 4. Describe the medical director’s tasks that contribute to the facility’s infection control program 5. Access current regulations and clinical guidelines that impact this area of medical direction. 6. Choose and utilize appropriate techniques and data sources for facility-wide monitoring of infectious disease. 07 Residents Rights 1. 2. 3. 4. 5. 08 Financial Issues 1. Explain the differences between the sources of Long Term Care funding. 2. Communicate effectively with the administrator concerning the expense and revenue aspects of the facility budget. 3. Define the nature of the Medical Director’s roles and responsibilities relative to financial issues in long term care facilities. 4. Identify issues related to documentation, coding and physician reimbursement in long term care. Enumerate basic categories of Residents Rights. Discuss factors that influence the ability of residents to exercise their rights. Describe common situations where Residents Rights are relevant. Discuss the prevention of and response to abuse and neglect. Compare and contrast the medical director’s role and the attending physician’s role in honoring Residents Rights. 10 CORE CURRICULUM ON MEDICAL DIRECTION Learning Objectives Part II Part II Overall Relate the training and typical tasks of the members of the multidisciplinary team and realize how each contributes to total resident care. Explain how the collection and use of data supports quality management and initiatives. Delineate the medical director’s responsibility in ensuring facility-wide ethical decision making. Employ communication strategies to learn the basic concepts of each family system and to address complex family situations. Analyze the content of the medical director’s contract to ensure that all elements are covered, including risk management and liability insurance. Establish policies, procedures, and tools that enhance care, quality management, and reduce facility risks. Assimilate the concepts of leadership, organizational culture, and values that enhance management and care processes. Develop an individualized action plan to implement new strategies or problem solutions at the site of practice. 09 Introduction to Medical Care Delivery Systems 1. 2. 3. 4. 10 Essential Health Information Tools in Medical Direction (MDS, RAI, Oasis, others) 1. 2. 3. 4. 5. 6. 11 Medical Director’s Report and Contract 1. 2. 3. 4. Describe the basic elements of systems theory. Discuss the characteristics of organization. List the types of care delivery systems to patients in Long Term Care Facilities. Describe the training and typical roles and functions of the members of the multidisciplinary team. 5. Value the contributions of the different members of the multidisciplinary care team. Trace the history and relevance of the MDS. Explain the process of data collection in creating the MDS. Utilize the MDS in the Resident Assessment Instrument for care planning. Describe how the MDS is utilized as a reimbursement tool. Demonstrate how the MDS is utilized by CMS for monitoring quality. Evaluate the application of additional data sets (Oasis, UDS-FIM, pharmacy and lab composite reports). 7. Recognize potential uses of MDS data for outcomes evaluations, research and quality management. Define the purpose and content of the Medical Director’s report. Describe the elements and content of the medical director’s contract. Ensure that all of the essential elements are in the participant’s contract. Discuss elements of risk management including liability insurance and anti-kickback provisions. 12 Personality Profiles 1. List the four domains of Myers-Briggs. 2. Explain the differences between the four domains and how they help create a personality profile. 3. Recognize the potential impact of the four domains may have on Medical Director's functions. 13 Employee Behavior 1. Describe situations in which employees may not know why and what they should be doing. 2. Explain why no positive consequences for the right thing could negatively influence employees’ behaviors. 3. Apply concept of Fourniers differential diagnosis to help correct negative behaviors. 11 CORE CURRICULUM ON MEDICAL DIRECTION 14 Medical Staff Oversight Learning Objectives 1. Explain rationale and discuss basic Medical Director responsibilities for Medical Staff oversight. 2. Describe models of Medical Staff organization and oversight, including non-physician staff members. 3. Define responsibility in credentialing and privileging. 4. Delineate issues and develop strategies to address medical staff issues concerning roles, functions or tasks, including non-physician staff. 15 Biomedical Ethics 1. 2. 3. 4. 16 Working with Families 1. 2. 3. 4. 5. 6. 17 Quality Management 1. Describe QA and TQM principles and tools. 2. Use QA and TQM tools to evaluate and enhance health professional and system performance. 3. Assist facility in developing and/or maintaining compliance program. 18 Risk Management 1. 2. 3. 4. 19 Systems Theory and Problem Solving 20 Governance 21 Committees Discuss basic principles (concepts) relevant to biomedical ethics. Identify key process steps in managing ethical issues. Apply key ethical principles and processes in various situations. Delineate Medical Director responsibilities in helping to ensure facility-wide ethical decision-making. 5. Use appropriate resources for good decision-making. 6. Discuss ethical considerations of research in vulnerable subjects. Define basic concepts of family systems, including boundary, structure, and culture. Relate chronic disease to patient and family relationships and interactions. Identify common patterns of family behavior that arise in the LTC setting. Discuss effective strategies to enhance physician communication with families. Employ appropriate strategies to deal with complex family situations. Delineate the Medical Director’s tasks that address family issues. Establish policies and procedures for an effective facility risk management program. Define the core elements contained within an incident report. Describe the relationship of unions and medical director. Identify risk management strategies to reduce medical director's liability. 1. Describe the process of problem solving. 2. Apply systems theory and medical direction tools and skills to problem solving in long-term care settings. 1. Describe relationships between Medical Directors and governing body, boards, administrators, and staff. 2. Illustrate basic governance arrangements. 1. Define the role and functions of committees and committee members. 2. Develop and define the role and functions and tasks of committees and committee. members, and the specific role the medical director will play. 12 CORE CURRICULUM ON MEDICAL DIRECTION Learning Objectives Define basic leadership and management principles. Compare and contrast the differences. List the skills helpful to exert leadership as a medical director in a long term care facility. Demonstrate two leadership skills which may be helpful in your facility. Understand the potential power sources in your facility. Describe how the behavior of leaders and managers create and define an organizational culture. 7. Apply an understanding of personality types to your leadership role and how it may influence team process. 22 Leadership 1. 2. 3. 4. 5. 6. 23 Integration of the Medical Director’s Role and Development of Individualized Action Plan 1. Synthesize the functions and tasks of the medical director to fulfill the role of the medical director. 2. Develop an individualized action plan. 3. Review the content, format and rationale of the Core Curriculum and agree to participate in the evaluation and follow-up of the course. 13 Core Curriculum on Medical Direction in Long Term Care Target Audience Medical directors practicing in any setting or combination of settings across the long term care continuum, including skilled nursing facilities, assisted living, CCRCs, hospice, and home care are encouraged to attend. Geriatric fellows in training who are considering the inclusion medical direction in their practices are also encouraged to attend. This course is the foundation for certification as an AMDA CMD. Taking the course does not make you a CMD. There are other requirements to complete. Contact AMDA at 800-876-2632 for a CMD Brochure outlining the details of certification as an AMDA CMD. Course Objectives The goal of this comprehensive course is to create a stronger sense of the leadership role of the medical director and to provide opportunities to hone skills and interact with peers. Following the conference, participants should be able to: Develop practical skills needed to fulfill the role and responsibilities of the medical director. Identify the unique aspects of the long term care environment that impact the medical director’s job. Describe the organizational responsibilities and dynamics of the medical director and the interdisciplinary team. Develop communication skills to deal with responsibilities for the interdisciplinary team, residents, and their families. Explain the resident care responsibilities of the medical director, including emergency care, quality management, family systems, and ethical considerations. Enhance leadership skills and team building towards a stronger role for the medical director with the interdisciplinary team. Develop human resource skills to deal with difficult situations and improve personal effectiveness in this area. Improve the medical director’s ability to learn and practice in the evolving environment of health care delivery. Apply newly acquired knowledge to daily facility and practice activities. Accreditation The American Medical Directors Association is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. CME Credit AMDA designates this educational activity for a maximum of 46 AMA PRA Category 1 Credit(s)TM. Physicians should only claim credit commensurate with the extent of their participation in the activity. 14 Certified Medical Director (CMD) Credit The AMDA Core Curriculum on Medical Direction has been approved for a maximum of 46 credit hours in medical direction. Credit hours may be applied toward certification as a Certified Medical Director in Long Term Care (AMDA CMD). The AMDA CMD program is administered by the American Medical Directors Certification Program (AMDCP). Physicians should only claim credit commensurate with the extent of their participation in the activity. AAFP Credit This program has been reviewed and is acceptable for up to 44.25 prescribed credits by the American Academy of Family Physicians. AGS Credit This program has been endorsed by the American Geriatrics Society. Credits earned from this activity may be counted toward the AGS Geriatrics Recognition Award. Tracking and Sign-In Sheets Attendees should sign in daily (morning and afternoon). All attendees seeking credit should track their participation on the tracking sheet that AMDA has provided. At the end of the course, keep the white copy for your records and return the yellow copy to AMDA, as you will not receive an additional certificate. AMDA Disclaimer Statement Participants understand that medical and scientific knowledge are constantly evolving. The views and treatment modalities of the authors are their own and may reflect innovations (including off-label or investigational use of medical products) and opinions not universally shared. Every effort has been made to assure the accuracy of the data presented in the context of accepted medical practice. Physicians should check specific details such as drug doses and contraindications, off-label uses, or other details in standard sources prior to clinical application. The views and treatment modalities of the authors are not those of the American Medical Directors Association (AMDA), but are presented in this forum to advance scientific and medical education. Requirement for Author Disclosure ACCME and AMDA policy requires everyone in a position to control the content of this educational activity to provide full disclosure of any affiliation or financial interest that is directly relevant to speaker’s presentation(s). In addition, speakers are required to disclose when references to pharmaceuticals, medical devices, or other therapeutic products used in treatments are “off-label” (not approved by FDA for the use described). Disclosure information is reviewed in advance to manage and resolve any conflict of interest that may affect the balance and scientific integrity of an educational presentation. Faculty Disclosure Information Faculty are required to provide verbal disclosure prior to each talk. If the learner perceives any bias toward a commercial product or service, please report this to AMDA staff. All program planners and faculty have provided full disclosure and report no relationships relevant to this course. 15 Core Curriculum on Medical Direction in Long Term Care Faculty Roster and Biographies Jeffrey B. Burl, MD, CMD (Course Chair) Sutton, MA jeffrey.burl@fallon-clinic.com Jeffrey Burl, MD, CMD is Medical Director of the Overlook Masonic Nursing and Rest Home and Clinical Director of the Fallon Clinic Division of Geriatrics. He is the director of the AMDA Core Curriculum as well as member of the education, finance and competency committees. Alva S. Baker, MD, CMD Sykesville, MD dr.alva.baker@grnmd.com Alva S. Baker, MD, CMD began caring for frail elderly patients in the long term care setting in 1972. He served as the Medical Director for Episcopal Ministries to the Aging (EMA) from 1980 until his retirement in June of 2009 as Vice President for Health and Wellness Services for EMA. He concurrently served as the Executive Director of The Copper Ridge Institute, wherein his focus of research and teaching has been on the care of persons with Alzheimer’s disease and other forms of dementia. He serves on the faculty of the Division of Geriatric Psychiatry and Neuropsychiatry of the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine. He is also serves on the Gerontology faculty at McDaniel College, where he holds the position of Director of The Center for the Study of Aging. Board certified in Geriatrics and in Hospice and Palliative Medicine, he serves on the Professional Advisory Committee of Carroll Home Care/Carroll Hospice, as an Associate Medical Director for Carroll Hospice, and on the Board of Directors of the Partnership for a Healthier Carroll County. Dr. Baker is an Emeritus member of the Medical Staff of Carroll Hospital Center in the Department of Internal Medicine. He has taught extensively about medical direction issues and continuous quality improvement to medical directors and staff at all levels in residential care facilities throughout the long term care continuum. He is a Certified Medical Director and a member of the American Medical Directors Association, for which he served as President in 2007-2008. Daniel Bluestein, MD, MS, CMD Norfolk, VA bluestda@evms.edu Daniel Bluestein, MD, MS, CMD, AGSF holds the Certificate of Added Qualification in Geriatrics and is Professor of Family & Community Medicine, Eastern Virginia Medical School. As Director of the Department's Geriatrics Division, he is responsible for geriatrics training offered to Family Medicine and Family Medicine-Internal Medicine Combined program trainees. He is also an AMDA-certified Medical Director at multiple long-term care facilities. Dr. Bluestein is faculty for the AMDA Core Curriculum, has presented at multiple AMDA and other national Geriatrics meetings and is the holder of two AMDA Foundation/Pfizer Continuous Quality Improvement awards. He is also a past chair of the ADMA Communications Committee. 16 J. Kenneth Brubaker, MD, CMD Mount Joy, PA jkbrubak@masonicvillagespa.org J. Kenneth Brubaker, MD, CMD completed his geriatric fellowship in 1989 and works fulltime as a geriatrician. During the past 20 years, Dr. Brubaker has worked as a medical director in several large CCRC in addition to caring for residents. Presently, he is the medical director of Masonic Village in Elizabethtown, PA and Willow Valley Retirement Communities in Willow Street, PA. Together the two facilities serve over 500 skilled residents, over 300 personal care residents, and several thousand independent living residents, In addition to running a 35 bed dementia unit, Dr. Brubaker serves as the PDA/OLTL for the PA Dept. of Aging /Office of Long Term Living and as a faculty member of the Lancaster General Geriatric Fellowship Program. David A. Brechtelsbauer, MD, CMD Sioux Falls, SD david.brechtelsbauer@usd.edu David A. Brechtelsbauer, MD, CMD is an Associate Professor in the Department of Family Medicine at The Sanford School of Medicine of The University of South Dakota, and an Associate Director at the Sioux Falls Family Medicine Residency. In addition to being Board Certified in Family Medicine, he holds a Certificate of Added Qualifications in Geriatric Medicine and is a Certified Medical Director. He was awarded the James Pattee Excellence in Education Award Presented by the American Medical Directors Association in March 2005. He is a past President of the American Medical Directors Association. Robert G. Kaplan, MD, CMD Longwood, FL drrkaplan@aol.com Robert G. Kaplan, MD FACP CMD is Board Certified in Internal Medicine with a CAQ in Geriatrics, and a Certified Medical Director. He has an extensive background in Long-Term Care, and serves as a Multi-Facility Medical Director and Attending Physician. He is a Board member of the Florida Medical Directors Association and currently President elect. Dr. Kaplan is a Fellow of the American College of Physicians, was a practicing Internist for approximately twenty years, and a former Chairman of the Department of Medicine and Medical Staff President of South Seminole Hospital in Longwood, Florida. A graduate of New York University and the University of Brussels School of Medicine, Dr. Kaplan completed his residency at the Genesee Hospital in Rochester, New York. Karyn Leible, MD, CMD Rochester, NY kpleible@gmail.com Karyn Leible, MD, CMD is an internist with a Certificate of Added Qualifications (CAQ) in Geriatrics. During her geriatric fellowship, she concentrated on long term care and palliative care medicine. She has practiced in 3 states, Colorado, Florida and Georgia. She has spent time doing clinical practice in academic medicine at Emory University in Atlanta as well as private practice in Colorado and Florida. Currently, she is in Rochester New York where she is Sr. VP of Medical Services for Jewish Senior Life. She is Immediate Past President for the American Medical Directors Association. Peter Winn, MD, CMD Oklahoma City, OK peter-winn@ouhsc.edu Peter Winn, MD, CMD is a Professor at the University of Oklahoma for the Department of Family Medicine and Adjunct Professor for the Department of Geriatric Medicine. Dr. Winn is Board Certified in Family Medicine in the United States and Canada and has CAQs in Geriatrics and Hospice. He is a Palliative Medicine Medical Director for the long term care (LTC) Unit at the Fountains at Canterbury and is the Medical Director for Mercy at Home Hospice. 17 Core Curriculum on Medical Direction in Long Term Care Commonly Used Acronyms and Terms in Long Term Care ACLS Advanced Cardiac Life Support ADE Adverse Drug Event ADL Activities of Daily Living ADR Adverse Drug Reaction AIMS Abnormal Involuntary Movement Scale AL / ALF / ALC Assisted Living / Assisted Living Facilities / Assisted Living Center ATLS Advanced Trauma Life Support BBA Balanced Budget Act BBRA Balanced Budget Refinement Act BLS Basic Life Support CCRC Continuing Care Retirement Community CF Conversion Factor CFR Code of Federal Regulations CMD Certified Medical Director (through AMDA) CME Continuing Medical Education CMN Certificate of Medical Necessity CORF Comprehensive Outpatient Rehabilitation Facility CPT Common Procedural Terminology – a system of codes for billing for physician services. CQI Continuous Quality Improvement CR Chemical Restraints DJD Degenerative Joint Disease DME Durable Medical Equipment DNR Do Not Resuscitate DON Director of Nursing DRGs Diagnosis Related Groups 18 ECF Extended Care Facility EMR Electronic Medical Record FQHMO Federally Qualified Health Maintenance Organization FPL Federal Poverty Level F-Tags A designation used by state survey agencies to identify particular tag sets within the state operation manual’s interpretative guidelines. HCPCS HCFA Common Procedural Coding System HCR Health Care Reform HEDIS Healthplan Employer Data and Information Set – An automated database for Managed Care; HEDIS is a set of standardized performance measures designed to ensure that purchasers and consumers have information to compare the performance of managed health care plans. H&P History and Physical HHC Home Health Care – Care provided to individuals in their homes. Patients must need a skilled service (nursing, PT, OT, ST) to qualify for Medicare home health benefit; also have to be homebound and need help only intermittently. If patient qualifies, patient can also get assistance from a home health aide. Hospice Program of palliative (comfort) care for persons who are dying. Medicare covers hospice services, which may be provided at home or in a hospital or SNF. Individual must be certified by physician as having less than 6 months to live. ICF Intermediate Care Facility IPO Insured Product Option LMRPs Local Medical Review Policies LTC Long Term Care LTCF Long Term Care Facility MCO Managed Care Organization MDS MICU Minimum Data Set – used for assessment and care, quality assurance and improvement, reimbursement, and survey process. Medical Intensive Care Unit MLP Midlevel Practitioner MSS Medical Social Services MSW Master of Social Work or Medical Social Worker NDC National Drug Code NF Nursing Facility – can be used to denote a nursing home that is not certified for Medicare (e.g. not a SNF) NF National Formulary 19 OASIS Outcome and Assessment Information Set (for home care patient) OBQI Outbound-Based Quality Improvement (used by Medicare certified home health care agencies to measure patient outcomes) OBRA Omnibus Budget Reconciliation Act OSCAR Online Survey Certification and Reporting PA Physician Assistant PASARR Preadmission Screening and Annual Record Review PDP Prescription Drug Plan (with Medicare Part D) PHO Physician-Hospital Organization POS Point of Service Post-acute care Services patients receive after an acute illness (usually entailing a hospital stay.) Can refer to SNF/NF and home health services, as well as other rehabilitation services. PPO Preferred Provider Organization PRIT Physicians Regulatory Issues Team PPS Prospective Payment System PSO Provider-Sponsored Organization PSRO Professional Standards Review Organization QA Quality Assurance QAAC Quality Assessment and Assurance Committee QI Quality Improvement, Quality Indicator, based on MDS data, or Qualified Individual (Medicare) QIO Quality Improvement Organization QM Quality Management or Quality Measure, based on MDS data QMB Qualified Medicare Beneficiary RAI Resident Assessment Instrument RAP Residential Assessment Protocol – used for decision making, care planning and implementation, and evaluation. RBRVS Resource Based Relative Value System RNAC Registered Nurse Assessment Coordinator RUGs Resource Utilization Groups RVUs Relative Value Units – components (e.g., physician work, practice expense, malpractice expense, etc.) used in calculating Medicare physician fee schedule. S&C Survey and Certification 20 SCU Specialized Care Unit SLMB Specified Low-Income Medicare Beneficiary SNF Skilled Nursing Facility – A nursing facility (or specially certified part of one) that participates in Medicare. SOAP Subjective Objective Assessment and Plan for progress notes SOM State Operations Manual (published by CMS) TQM Total Quality Management UM Utilization Management UR Utilization Review 21 Commonly Used Acronyms for Medical Organizations AAAHC Accreditation Association for Ambulatory Health Care AAFP American Academy of Family Physicians AAHCP American Academy of Home Care Physicians AAHPM American Academy of Hospice and Palliative Care AAHSA American Association for Homes and Services for the Aging – represents nonprofit providers of nursing facilities, home health agencies, assisted living communities, and continuing care retirement communities. ACHCA American College of Health Care Administrators ACP – ASIM American College of Physicians – American Society of Internal Medicine ADA American Dietetic Association ADC Alzheimer’s Disease Center ADEAR Alzheimer’s Disease Education and Referral Center AHCA American Health Care Association – represents for-profit owners of nursing facilities and assisted living facilities. AHRQ Agency for Healthcare Research and Quality AIR American Institute of Research ALFA Assisted Living Federation of America ALZ ASSN Alzheimer’s Association AMDCP American Medical Directors Certification Program APIC Association for Professionals in Infection Control and Epidemiology ASCP American Society of Consultant Pharmacists BQC, BQA Bureau of Quality Compliance or Assurance CDC Centers for Disease Control CERTs Centers for Education & Research on Therapeutics CMS Centers for Medicare and Medicaid Services (formerly HCFA) DEA Drug Enforcement Authority DHHS DSS Department of Health and Human Services Department of Social Services FDA Food and Drug Administration FEHBP Federal Employees Health Benefit Program 22 GSA Gerontological Society of America HCFA Health Care Financing Administration (now CMS) – agency that administers Medicare and Medicaid. HHS Health and Human Services JCAHO Joint Commission on Accreditation of Healthcare Organizations MedPAC Medicare Payment Advisory Commission MSO Management Services Organization NADONA National Association of Directors of Nursing Administration NAGNA National Association for Geriatric Nurse Aides NAIC National Association of Insurance Commissioners NANDA North American Nursing Diagnosis Association NCCNHR National Citizens Coalition for Nursing Home Reform NCI National Cancer Institute NCQA Managed Care Association NCQA National Committee for Quality Assurance NFCA National Family Caregivers Association NIH National Institutes of Health NIMH National Institutes of Mental Health NLN National League for Nursing NPDB National Practitioner Data Bank NQF National Quality Forum OCI Office of the Commissioner of Insurance OIG Office of the Inspector General OMB Office of Management and Budget OPHC Office of Prepaid Health Care OSHA Occupational Health and Safety Administration OTA Office of Technology Assessment PRO Peer Review Organization (also known as QIO) QIO Quality Improvement Organization SHEA Society for Healthcare Epidemiology of America USP United States Pharmacopeia USPHS United States Public Health Service 23 ORIGINAL STUDIES Impact of Medical Director Certification on Nursing Home Quality of Care Frederick N. Rowland, PhD, MD, CMD, Mick Cowles, BA, MS, Craig Dickstein, BA, MS, and Paul R. Katz, MD, CMD Objective: This study tests the research hypothesis that certified medical directors are able to use their training, education, and knowledge to positively influence quality of care in US nursing homes. Design: F-tag numbers were identified within the State Operations Manual that reflect dimensions of quality thought to be impacted by the medical director. A weighting system was developed based on the ‘‘scope and severity’’ level at which the nursing homes were cited for these specific tag numbers. Then homes led by certified medical directors were compared with homes led by medical directors not known to be certified. Data/participants: Data were obtained from the Centers for Medicare & Medicaid Services’ Online Survey Certification and Reporting database for nursing homes. Homes with a certified medical director (547) were identified from the database of the American Medical Directors Association. Measurements: The national survey database was used to compute a ‘‘standardized quality score’’ (zero representing best possible score and 1.0 representing Since the introduction of the concept of nursing home medical directors in the 1970s there have been multiple papers, guidelines, and books published on the role of the medical director and how this should affect the quality of care in the nursing home. Section of Geriatric Medicine, Saint Francis Hospital and Medical Center, Hartford, CT (F.N.R.); Mercy Community Health, West Hartford, CT (F.N.R.); Cowles Research Group, McMinnville, OR (M.C.); Tamarack Professional Services, LLC, Caratunk, ME (C.D.); Division of Geriatrics/Aging, University of Rochester School of Medicine and Dentistry, Rochester, NY (P.R.K.) The authors have no conflicts of interest regarding this article. Address correspondence to Frederick N. Rowland, PhD, MD, CMD, Department of Medicine, Section of Geriatric Medicine, Saint Francis Hospital and Medical Center, 114 Woodland Street, Hartford, CT 06105–1299. E-mail: frowland@stfranciscare.org Copyright Ó2009 American Medical Directors Association DOI:10.1016/j.jamda.2009.05.012 ORIGINAL STUDIES average score) for each home, and the homes with certified medical directors compared with the other homes in the database. Regression analysis was then used to attempt to identify the most important contributors to measured quality score differences between the homes. Results: The standardized quality score of facilities with certified medical directors (n 5 547) was 0.8958 versus 1.0037 for facilities without certified medical directors (n 5 15,230) (lower number represents higher quality). When nursing facility characteristics were added to the regression equation, the presence of a certified medical director accounted for up to 15% improvement in quality. Conclusions: The presence of certified medical directors is an independent predictor of quality in US nursing homes. (J Am Med Dir Assoc 2009; 10: 431–435) Keywords: Certified medical director; quality of care; medical director; nursing facility; skilled nursing facility There has, however, been no clear quantification of the impact that a well-trained medical director can have on the quality of care within a facility. The official mission statement of the American Medical Directors Certification Program (AMDCP) is to ‘‘.advance physician leadership.thereby enhancing quality of care.’’1 Since its inception in 1991, the AMDCP has certified more than 2500 medical directors. The certification process follows an ‘‘experiential’’ model that incorporates existing mechanisms such as fellowship programs, board certification, continuing medical education programs (offered by major provider organizations), courses in medical direction (approved by AMDCP), and other continuing education programs. Familiarity with the medical director certification process leads to the expectation that medical director certification is positively correlated with quality of care. Although such a correlation is commonly and reasonably asserted, we have found nothing in the literature empirically demonstrating such a relationship. Rowland et al 431 24 This study tests the hypothesis that certified medical directors are able to use their training, education, and knowledge to positively influence quality of care in US nursing homes. The alternate hypothesis (or null hypothesis) is that certification makes no appreciable difference to nursing home quality of care. This project was granted institutional review board (IRB)exempt status by the IRB of Wright State University via the American Medical Directors Research Foundation. The project was sponsored by generous grants from the American Medical Directors Certification Program and AMDA state chapter contributions. METHODOLOGY FINDINGS F tags from the State Operations Manual2 (N 5 27) were identified that appear to reflect dimensions of quality potentially directly impacted by the medical director. These were chosen by consensus of the research team including the authors, AMDCP staff, and the AMDCP Executive Committee on the premise that these were areas of quality potentially influenced by medical director activity. A weighting scheme based on the ‘‘scope and severity’’ level at which the nursing homes were cited for these specific tag numbers was also developed. This was constructed to emphasize serious, widespread, or patterns of deficiencies. It was thought that a better prepared medical director would be able to reduce the incidence of deficiency citations for these 27 specific F tags, or, at a minimum, reduce the scope and severity level at which they were cited. A listing of the 27 F tags and our scope and severity–based weighting scheme is listed in Appendix 1. The weighting scheme is similar, though not identical, to that used by the Nursing Home Compare Five-Star Rating guide.3 Using the Centers for Medicare & Medicaid Services’ Online Survey Certification and Reporting (OSCAR) database as of March 2008, a ‘‘raw quality score’’ was computed, and a ‘‘standardized quality score’’ for all 15,777 certified nursing homes that were in operation in the United States in March 2008.4 The raw quality score was computed by summing the weights of the relevant deficiency citations. We then divided the raw quality score by the state average raw quality score to yield a standardized quality score. Standardization of the quality score is necessitated by wide state-to-state variation in the survey process. For example, New Jersey nursing home surveys result in an average of 4 total deficiencies per survey, whereas in neighboring Delaware the comparable average is 13.5 Dividing the raw quality score by the state average ‘‘standardizes’’ the score, creating a measure that is comparable across states. Note that lower adjusted quality scores denote better quality, and an adjusted quality score of unity denotes average quality. Records of the American Medical Directors Association (AMDA) were then used to identify 547 nursing homes that had certified medical directors during the year immediately preceding and during the survey contained in our data capture. The first step was to compare the average standardized quality score in facilities with certified medical directors to those without certified medical directors. A ‘‘t test’’ was then computed to evaluate the degree to which the difference between the averages was statistically significant. Finally, other variables were considered that could also affect quality, and multiple regression analysis was used to better understand the relationship between medical director certification and quality of care. 432 Rowland et al As shown in Table 1, the average standardized quality score (SQS) in facilities with certified medical directors was 0.8958 compared with 1.0037 for facilities without certified medical directors. Recall that lower numbers represent better quality, with zero representing the best possible score. The difference of 0.1079 represents a 12% improvement in quality associated with the presence of a certified medical director, and the t test indicated that the difference is statistically significant at the 98% level. It was suspected, however, that other nursing home characteristics are correlated with quality, such as facility size, class of ownership, case mix, staffing, and urban/rural status. Smaller facilities should tend to have fewer deficiencies because of fewer opportunities for errors, and not-for-profit facilities are known to have better surveys than for-profit facilities.5 The higher case mix associated with more medically complex cases might result in more deficiencies, higher staffing would be expected to result in fewer deficiencies, and rural facilities might have better surveys than urban facilities. The urban/rural impact on quality, if there is one, might more accurately be associated with size or staffing differences between urban and rural nursing homes. There appears to be significant potential for the statistical relationships hypothesized in the preceding paragraph to confound the initial findings reported in Table 1. For example, what if facilities with certified medical directors are more likely to be small, or more likely to be not-for-profit? If that were the case, then the variation in quality of care that is attributed to medical director certification in Table 1 might in fact be attributable to these other factors. Stepwise multiple regression analysis was used to help determine if this might be the case and to better understand the relationship between quality and medical director certification. Data from the best specified equation are reported in Table 2. It was found that the strongest predictors of adjusted quality were whether or not the nursing home had a certified medical director, whether or not the total number of beds in the facility was greater than 99, whether or not it was a proprietary (for-profit) facility, and the number of registered nurse (RN) staffing hours per patient day. Recall that the average adjusted quality score is 1.0000 and that lower numbers reflect better quality. Thus, the Table 1. Average Standardized Quality Score With and Without a Certified Medical Director (CMD) With CMD (n5547) Without CMD (n515,230) Difference # Difference % 0.8958 1.0037 0.1079 12.05 JAMDA – July 2009 25 Table 2. Regression Equation Predicting Standardized Quality Score Dependent Variable: Adjusted Quality Score Number of observations read Number of observations used Number of observations with missing values 15777 15618 159 Analysis of Variance Source Model Error Corrected total DF Sum of Squares 4 15613 15617 562.03592 37431 37993 Root MSE Dependent mean Coefficient of variation Mean Square 140.50898 2.39739 1.54835 1.00559 153.97489 F Value Pr.F 58.61 \.0001 R-Square Adj R-Square 0.0148 0.0145 Parameter Estimates Variable DF Intercept Certified medial director present Beds .99 For profit Registered nurse hours per patient day 1 1 1 1 1 Parameter Estimate 0.98780 0.14705 0.22309 0.08987 0.24987 estimated regression coefficient of –0.14705 for the certified medical director variable indicates that, holding other predictors constant, the presence of a certified medical director will improve quality by about 15%. Other results from the regression equation were consistent with stated preconceptions, ie, larger facilities, proprietary facilities, and facilities that staff fewer RN hours per patient day tend to have poorer quality. Urban/rural status and case mix are not included in the equation as they did not improve equation specification. DISCUSSION For the first time, this study demonstrates that the certified medical director has a measurable positive effect on the quality of care provided in facilities in which they serve. In 1975, regulations were promulgated that required skilled nursing facilities to have a medical director. In response to this, AMDA was formed in 1977 to organize the medical directors and provide a venue for education of the medical directors in their role and responsibilities. Numerous articles (representative articles in references) have been written since that time about the role of the medical director.6–10 Articles have been written on specific problems in which the medical director can and should make a difference.11–13 Textbooks on the role of the medical director14,15 and long-term care medicine16,17 have been published. To the best of our knowledge, only one has made an attempt to show via survey of medical directors and administrators in Maryland that requiring medical director training makes a positive difference in the quality of medical directorship provided.18 In that study, which reports on a survey of medical directors and administrators following the institution of mandatory medical director education, there was consensus that the relationship between the medical director and other administrators in ORIGINAL STUDIES Standard Error 0.03461 0.06783 0.02546 0.02744 0.02918 T Value 28.54 2.17 8.76 3.7 8.56 Pr./t/ \.0001 .0302 \.0001 .0011 \.0001 the nursing home was improved, that the medical director spent more time in the facility working on system issues, and that the medical director spent more time with the administrator reviewing the care provided. In contrast, the present study uses a comparison of actual survey data from the facilities. The current study was initiated in an attempt to demonstrate whether the presence of a certified medical director made a measurable difference in the quality of care provided within long-term care facilities. The results support the conclusion that the presence of a certified medical director makes an appreciable and positive difference on the quality of care provided within long-term care facilities. The data also support the premise that there are other important factors determining the quality of care provided. OTHER FACTORS AFFECTING RESULTS There is great confidence that all 547 nursing homes that were flagged as having certified medical directors during the study period actually did have certified medical directors because all certified medical directors identified their facility as where they worked in their medical director role for AMDA records in the time frame immediately before this study. The comparison group of 15,230 facilities that are treated as not having a certified medical director may actually contain facilities that may have had a certified medical director during all or part of the study period. This is likely, because of the 2500 certifications awarded, it is estimated via AMDA records that approximately 1500 of these individuals are still working. If the truth is that certified medical directors are associated with higher quality, then including facilities in the control group that actually had certified medical directors would tend to reduce the difference between the 2 comparison groups. Thus, if there were inadvertently Rowland et al 433 26 included facilities in the comparison group that had certified medical directors, then the true difference between the certified and noncertified groups was larger than what is reported, ie, the research results are even more robust than what is reported. The probability values reported in Table 2 are for a 2-tailed t test. We could argue on theoretical grounds that the 1-tailed test is more appropriate. Interpreting our t ratios using a 1-tailed test would also make our results more robust, ie, double the level of statistical significance for each predictor variable. Of note on the statistical analysis of the linear regression model is that the multiple correlation coefficient (R squared) is relatively low (0.0148); however, it needs to be placed in the context that the goal of this study was to test whether the presence of a certified medical director made a positive impact on the quality of care in that nursing home, not to explain the total variation in the quality measured. Thus, the magnitude of the partial correlation coefficient associated with the certified medical director variable (–0.14705) and its associated level of statistical significance (.0302) are of much greater importance than the absolute value of the multiple correlation coefficient. Other factors that theoretically could bias the outcome are that 2 of the authors are currently certified medical directors and medical directors of facilities included in the database, however it is doubtful that 2 individual homes would bias the overall results in comparison with either the 547 identified facilities with a certified medical director or the 15,230 other facilities. A potentially more important variable is that many AMDA members have trained in geriatric fellowship programs and have certification in geriatric medicine. Of the certified medical director–led facilities in this study, 18% (101 of the 547) are led by medical directors with geriatric fellowship training. We did not attempt to separate out the contribution of this training in the current project, but a recent survey study examined barriers to care and visit time expectations, which revealed that geriatric-trained physicians may have a higher level of expectation in their care of long-term care patients.19 Currently, all long-term care facilities are required to have a physician identified as medical director. The data now reported suggest that there is a clear and measurable positive effect on quality if that medical director is a certified medical director. This may have policy implications in all of long-term care. Because the certified medical director designation indicates a minimum level of experience and education in medical director management and clinical geriatric medicine, it suggests that every long-term facility and program should have a certified medical director or the equivalent. An alternate explanation is that certified medical directors are a self-identified group of dedicated, experienced individuals who are willing to be held accountable as longterm care providers and leaders, and that they would be so whether or not they had attained recognition as a certified medical director. Whatever the reason, our patients deserve the best of all of us. 434 Rowland et al CONCLUSION This research demonstrates that the presence of a certified medical director in a facility makes an appreciable positive difference in the quality of care provided in that facility. The data also identify other factors—small facility size, not-for-profit status, and higher RN hours per patient day—as important determinants of higher quality offered by a facility. It is hoped that this will lead to further recognition of the knowledge and skills of trained medical directors, and encourage all medical directors to work to attain and improve these skills. REFERENCES 1. AMDA. Certified Medical Director in Long Term Care (AMDA CMD). Available at: http://www.amda.com/certification/overview.cfm. Accessed June 17, 2009. 2. Interpretive Guidelines for Long-Term Care Facilities. Available at: http://cms.hhs.gov/manuals/Downloads/som107ap_pp_guidelines_ltcf. pdf. Accessed June 17, 2009. 3. NursingHome Compare. Design for Nursing Home Compare Five-Star Quality Rating System: Technical Users Guide. January 2009. Available at: http://www.cms.hhs.gov/CertificationandComplianc/13_FSQRS.asp. Accessed June 17, 2009. 4. Obtained from the files of Cowles Research Group. Available at: http:// www.longermcareinfo.com/about_oscar.html. Accessed June 17, 2009. 5. Cowles CM. Nursing Home Statistical Yearbook: 2007. McMinnville, OR: Cowles Research Group; 2008. pp.70, 72–73. 6. American Medical Directors Association. Roles and responsibilities of the medical director in the nursing home: position statement A03. J Am Med Dir Assoc 2005;6:411–412. 7. Schnelle JF. Total quality management and the medical director. Clin Geriatr Med 1995;11:433–448. 8. Schnelle JF, Ouslander JG. CMS guidelines and improving continence care in nursing homes: The role of the medical director. J Am Med Dir Assoc 2006;7:131–132. 9. Zimmer JG, Watson NM, Levenson SA. Nursing home medical directors: Ideals and realities. J Am Geriatr Soc 1993;41:127–130. 10. Smith RL, Osterweil D. The medical director in hospital-based transitional care units. Clin Geriatr Med 1995;11:373–389. 11. Colon-Emeric CS, Casebeer L, Saag K, et al. Barriers to providing osteoporosis care in skilled nursing facilities: perceptions of medical directors and directors of nursing. J Am Med Dir Assoc 2005;6:S61–S66. 12. Richards CL Jr.. Preventing antimicrobial-resistant bacterial infections among older adults in long-term care facilities. J Am Med Dir Assoc 2005;6:144–151. 13. Munir J, Wright RJ, Carr DB. A quality improvement study on calcium and vitamin D supplementation in long-term care. J Am Med Dir Assoc 2006;7:305–309. 14. Pattee JJ, Otteson OJ. Medical Direction in the Nursing Home: Principles and Concepts for Physician Administrators. Minneapolis, MN: North Ridge Press; 1991. 15. Levenson SA, editor. Medical Direction in Long-Term Care: A Guidebook for the Future. 2nd ed. Durham, NC: Carolina Academic Press; 1993. 16. Katz PR, Calkins E, editors. Principles and Practice of Nursing Home Care. New York: Springer Publishing; 1989. 17. Ouslander JG, Osterweil D, Morley J. Medical Care in the Nursing Home. 2nd ed. New York: McGraw-Hill; 1997. 18. Boyce BF, Bob H, Levenson SA. The preliminary impact of Maryland’s medical director and attending physician regulations. J Am Med Dir Assoc 2003;4:157–163. 19. Caprio TV, Karuza J, Katz PR. Profile of physicians in the nursing home: Time perception and barriers to optimal medical practice. J Am Med Dir Assoc 2009;10:93–97. JAMDA – July 2009 27 Appendix 1 F-tags Included in Standard Quality Scores The following list of F-tags was determined by consensus to be those most likely to be directly influenced by the medical director. F-Tag Area of Medical Direction 202 221–222 223 280 281–282 309 314 319–320 323–324 325 329 385, 386, 387, 388, 390 441, 442, 443, 444 492 Identify appropriate ways to minimize avoidable transfers Restraints: Policies and procedures; alternatives to use Freedom from abuse Train attending physicians to help staff develop resident care plan Medical direction—additional duties QA relative to MDS Pressure ulcers Access to mental health treatment Minimizing and reporting accidents Weight loss and nutrition Unnecessary drugs Quality assurance issues around physician performance Infection control Compliance with federal, state, and local laws and regulations; physician oversight; additional duties (see also 281–282) Medical direction Establish and implement a relevant facility-wide quality assurance program, including a QA committee 501 520 At the same time, the following weighting scale based on scope and severity was approved: Scope and Severity Designation Weight A – isolated event, no actual harm B – possible pattern, no actual harm C – widespread, no actual harm D – isolated, no actual .minimal harm, no immediate jeopardy E – possible pattern, no actual .minimal harm, no immediate jeopardy F – widespread, no actual .minimal harm, no immediate jeopardy G – isolated, actual harm, no immediate jeopardy H – pattern, actual harm, no immediate jeopardy, substandard care I – widespread, actual harm, no immediate jeopardy, substandard care J – isolated, immediate jeopardy, substandard care K – pattern, immediate jeopardy, substandard care L – widespread, immediate jeopardy, substandard care deleted, not significant to our study 0 1 1 2 2 3 10 10 15 20 20 ORIGINAL STUDIES Rowland et al 435 28 29 01 Introduction 01 Course Introduction Welcome Core Curriculum on Medical Direction 2 1 Learning Objectives Delineate the content, format and rationale of the Core Curriculum. Define roles, functions and tasks as they apply to Medical Direction. Describe the behavioral expectations for the participants after the course, including the development of a personalized action plan. Share data about perceptions of participants’ current behavior. The Core Curriculum 3 4 Rationale for Curriculum Curriculum Design Physicians need to master a basic core of facts needed to work effectively in long term care as administrators. Two parts Part I – 2 days Experiential learning of attitudes and skills needed to function effectively as a medical director will optimize the performance of the physician serving in that capacity and improve the quality of care for the residents in the facility that s/he serves. 5 Didactic sessions covering basic factual information. Introduction of small group session as learning tool. Part II – 4 days Extensive use of experiential interactive small group sessions. Systems theory, leadership and management Developing behavioral models of effective medical direction. 6 30 01 Introduction Background of AMDA History of Core Curriculum AMDA was formalized as an organization in 1977, grown from a handful to over 6000. Basic Mission: Improve the quality of care of residents in the long term care continuum. Providing training in administrative medicine for medical directors in long term care. Certified Medical Director Program (AMDCP) In mid-1980’s, Dr. James Pattee at the University of Minnesota created the role and functions for Medical Directors. Dr. Pattee provided programs to teach these concepts (3 weekends, 60 hours). Core curriculum conference by AMDA in early 1990’s restructured this information into 36 hours of education in three sessions (Modules A, B, C). 7 History of Core Revision Faculty Disclosures AMDA embarked on project to re-evaluate the educational programs which taught the Curriculum. 8 All faculty have stated there are no disclosures to be made that are pertinent to this course. October, 2001 – initial workgroup meeting Additional work by experienced medical directors and Core faculty, with AMDA staff and educational consultant assistance Result: Redesign of education model, sequencing of topics, coordination of content, increase in contact hours to 46. 9 Participant Outcome 10 Resources The goal of this curriculum and this educational model is that at the end of this week, the participant will… Understand and utilize the knowledge, skills and attitudes needed to effectively fulfill the roles, functions and tasks of the Medical Director. 11 Course syllabus – slide sets Exercise workbook – ‘handouts’ you will need for class work in this room and in small groups Resources – additional resources in Adobe Acrobat document (.pdf) format. 12 31 01 Introduction Roles, Functions, and Tasks Role Definition The set of behaviors an organizational member is expected to perform and that he/she feels obligated to perform. Role of the Medical Director varies. Situation specific. Dependent on individual’s knowledge and skills and facility culture and needs. 13 14 Role Key Roles Medical Director is involved at all levels of patient care. Serves as the clinician who oversees and guides care. Leader to help define a vision of quality improvement. Direct supervisor of the medical practitioners. Operations consultant for day to day issues. Physician Leadership: Responsible overall care and clinical practice in the facility. Clinical Leadership: Applies clinical and administrative skills to help guide facility in providing care. 15 Key Roles 16 Function Quality of care: Helps the facility develop and manage both quality and safety initiatives. Education, information and communication: Provides information that helps others understand and provide care. Definition Functions of the Medical Director. 17 Major domains of activity within the role. Statements of responsibilities of the Medical Director. 18 32 01 Introduction Functions – Pattee JJ, Otteson OJ. Medical Direction in the Nursing Home - Principles and Concepts for Physician Administrators. 1991. Minneapolis, Minnesota: North Ridge Press Functions – The Medical Director . . . The Medical Director… 1. Participates in administrative decision making and recommends and approves policies and procedures. 2. Organizes and coordinates physician services and services provided by other professionals as they relate to patient care. 3. Participates in the process to ensure the appropriateness and quality of medical care and medically related care. 4. Participates in the development and conduct of educational programs. 5. Participates in the surveillance and promotion in the health, safety, and welfare of employees. 6. Helps articulate the long term care facility’s mission to the community. 19 20 Functions – Tasks The Medical Director . . . 7. Participates in establishing policies and procedures for assuring that the rights of individuals (resident, staff members, and community members) are respected. 8. Acquires, maintains, and applies knowledge of social, regulatory, political, and economic factors that relate to patient care services. 9. Person directed care. 21 Definition Specific activities used to carry out a function. Tasks Specific activities performed by the medical director to fulfill functions. 22 Tasks Function and situation specific. Timely and accurate completion of tasks ensures fulfillment of related function. Handout: Reference listing of common Medical Director tasks. 23 24 33 01 Introduction A System of Care A system is a unity of interrelationships and interactions. Events in one part of the system impact other parts of the system. 25 26 You Are Part of a System of Care! You Are Part of a System of Care! You have great influence and power in these environments by your presence that is derived from: Your influence and power extend as far as you wish to exert it! For example: Nutritional practices Admission standards Skin care protocols Quality assurance plan and emphasis Team building Pharmacy practices Professional expertise Personality (leadership ability) Demonstrated interest Title Ethical behavior (or lack of it) 27 28 Introduction to Medical Care Delivery Systems Introduction to Medical Care Delivery Systems There will be an explicit and deliberate effort to incorporate the principles of systems theory and systems thinking throughout the course. 29 Systems theory will be utilized to: Examine typical care delivery processes in LTC. Understand the roles, functions, and tasks of the Medical Director, as well as other members of the LTC interdisciplinary team. 30 34 01 Introduction Becoming a Leader Becoming a Leader Medical Directors by their position are de-facto leaders. We are unique in our facilities. We need to start to become leaders in our facilities. Curious and risk takers Concentrate at work Learn from adversity 31 Becoming a Leader Credibility Knowledge Ego Strength Have a sense of humor 32 Becoming a Leader Actions that exemplify desired values between individual and organizational values. Actions that communicate the presence of predictability, honesty, and concern. 33 Becoming a Leader 34 Resource Disk Actions that indicate a concern in followers’ interests as they relate to work, career, family, and extramural activities. Actions that indicate that leader’s interest in self-knowledge and selfdevelopment. Roles and Responsibilities of the Medical Director in the Nursing Home AMDA position statement 35 JAMDA articles: Relevant to the sessions presented through the week. 36 36 35 01 Introduction Behavioral Outcomes Individualized Action Plan Expectations Be an ‘active learner’ in the ongoing process of developing the knowledge, skills and attitudes needed in order to be an effective medical director. Develop a personalized ‘action plan’ of behavior changes and commitment for implementation upon return to work setting. Commitment to participate in ‘outcomes’ evaluation six months after completion of the course. 37 Individualized Action Plan From what you will learn in this course and using the worksheet: 38 Individualized Action Plan For each task, list the steps you will need to follow in order to implement the task. Create two separate IAP’s by the end of the course. For each chosen IAP you will choose one function and specify at least two tasks that you are presently not doing and that you will do upon your return. For each step, list the challenges or perceived barriers to implementation and what methods you will use to overcome them. As you proceed through the week think about your strengths and opportunities at your facility. Then choose two that you feel would be doable given your time and commitment. Feel free to ask the faculty for advice on a function\tasks. 39 Outcomes Evaluation Individualized Action Plan Six months from course completion, AMDA will send you a survey. 40 We are looking for you to respond to the survey concerning: Ability to implement plan. Usefulness of information learned. Behavioral changes experienced and projected. 41 When you complete and mail back the 6 month course survey, you will receive an additional 20 CME’s that may be used toward CMD requirement. Your IAP need not have been completed or successful to be eligible for the CME’s. 42 36 01 Introduction Outcomes Evaluation Distance view of course content: What was irrelevant? What was overdone? What could be added to course content? 1.1 – 1.12 43 44 37 02 Overview Session Objectives 02 Overview of Long Term Care: Past, Present and Future Core Curriculum on Medical Direction Review the demographics of aging in the USA. Discuss the evolution of long term care (LTC). Describe the LTC continuum of care and the key programs and organizations that provide that care. Review emerging challenges to LTC. Use your CMD skills to diagnose and treat your nursing facility! 2 Overview of Long Term Care Part I Demographics of Older Persons Part II Why Long Term Care Part III Payors for Long Term Care Part IV Industry Trends in Long Term Care Part V Using Your CMD Skills 2.1 – 2.6 4 Demographics – Number of Older Americans Age and Gender of the Medicare Population The proportion of women increases among those 85 and older. 17.9 million Enrollees (millions) 20 Females 18 Males 16 54% 14 12.6 million 12 10 8 59% 5.5 million 6 4 2 44% 4.7 million 46% 41% 56% Under 65 5 71% 29% 0 65-74 75-84 85+ Note: Fifty-six percent (23 million) of all Medicare beneficiaries are female; 44% (18 million) are males. Data reflect Medicare beneficiaries ever enrolled in the program during the year. Source: CMS, Office of Research, Development, and Information: Data from the Medicare Current Beneficiary Survey (MCBS) 2000 Access to Care File. 6 38 02 Overview Indicator 37 – Residential Services Where Medicare Beneficiaries Live For the six percent of beneficiaries living in long-term care facilities, most live in nursing homes but some live in assisted living/retirement homes or other facilities. Community with Skilled Nursing Facility 2.2% Community 91.4% LongTerm Care Facilities Other 0.5% Assisted Living / Retirement Homes 1.5% Nursing Homes 4.4% Note: Assisted Living/Retirement Home also includes Domiciliary Care Homes, Board and Care Homes, and Independent Living Units. All of these arrangements offer some level of assistance to the beneficiary. “Other” includes mental health facilities, mentally retarded/mentally disabled facilities and other unclassified facilities. Source: CMS, Office of Research, Development, and Information: Data from the Medicare Current Beneficiary Survey (MCBS) 2000 Cost and Use File. 7 Nursing Facility Population By Age 8 Transitions of Care: Revisited 3/10 3/10 Home 1/10 1st NH Admission 3/10 3/10 8/10 Acute Hospital Repeat NH Admission 6/10 1/10 4/10 9 Death 4/10 Kane RL, Ouslander JG, and Abrass IB: Essentials of Clinical Geriatrics, 2nd ed. New York, McGraw-Hill, 1989, Data source: Lewis MA, Cretin S, and Kane RL: The natural history of nursing home patients. Gerontologist 25:38210 388, 1985 The “Language” of Geriatrics Part II: Why Long Term Care? ADLs (Activities of daily living) The Issue: Progressive Loss of Abilities in the Last Years of Life 11 Ambulation Bathing Dressing Grooming Transferring Toileting Eating IADLs (Instrumental activities of daily) Transportation Phone use Shopping Preparing Meals Housework Taking Medications Personal Finances 12 39 02 Overview Percentage of People By Age and Sex Needing Assistance with Everyday Activities: Long Term Care Developed to meet these chronic needs. Definition: Health, social and personal services provided to the chronically ill and disabled of any age over an extended period of time in a variety of settings. Percentage Needing Assistance Source, CMS 13 14 Long Term Care Continuum Long Term Care Evolved from welfare system, not health system. 1965, Medicaid and Medicare legislation enacted- health care requirements were first introduced. Today, in nursing homes Medicaid pays 50 - 66% of costs Medicare pays 10% of costs Originally, care given by non professionals with little training- we have come a long way!! 15 Chronic Disease Hospitals Institutional Services Chronic Disease Hospitals Inpatient Rehab Units Nursing Facilities Skilled Nursing Facilities Subacute Care Units Respite Care Community Based Services Home Health Care Senior Centers Adult Day Care PACE Hybrid Services Assisted Living Hospice Case Management Assessment Programs 16 Inpatient Rehab Units Require more intensive medical & nursing care than NF or SNF, less than acute hospital. Ventilator Multiple complex wounds ESRD Multiple IVs Typical LOS around 30 days 17 Stay of many weeks. Must be able to do 3 hours of rehabilitation a day. Goal is to go “home.” 18 40 02 Overview Nursing Facilities (NF) Meet eligibility requirements for Medicaid reimbursement. Custodial Care Skilled Nursing Facilities (SNF) Formerly known as: Intermediate Care Facilities (ICFs) Definition: Treatment and continuing observation and assessment of the medically stable and unstable chronically ill. Must meet criteria for Medicare reimbursement. 19 20 Skilled Nursing Facilities (SNF) Skilled Nursing Facilities (SNF) Free standing or part of a NF as a wing or beds on a regular unit. Medicare Covered Stay (10%) Max. 100 days 20 days no copay, 80 copay Payor Medicare Part A / Managed Care Requires 3 day hospital stay (within 30 days for the same diagnosis) Requires skilled treatment. PPS Effective July 1, 1998 Per diem based on resource utilization Covers room, medications, diet, nursing services, medical supplies, DME, meds 21 Qualifying Needs for SNF PT/OT/Speech at least one hour a day Wound care Subacute Care Units Hospital Based PEG feedings Chronic oxygen therapy Dialysis Patients Diabetes monitoring 22 Non- Hospital Based (in NF or free standing) 23 Usually <20 day length of stay Same rules and regs as NFs Previously reimbursed significantly more than non hospital subacute units in SNFs when cost based systems was the norm. Physicians use NF CPT codes Same rules and regs as NFs Physicians use NF CPT codes 24 41 02 Overview Respite Care Designed to aid the caregivers. Short stays Often assisted living or nursing home facilities. Predominantly private pay (as well as hospice.) Nursing Facility Residents All Nursing Facility Residents "Short Stayers" (1 - 6 Months) Terminally Ill Cognitively Impaired Short Term Rehab Cognitively & Physically Impaired Subacute Physically Impaired (After Kane RL, Ouslander JG, and Abrass IB: Essentials of Clinical Geriatrics, 2d ed. New York, McGraw-Hill, 1994) 25 Nursing Facilities "Long Stayers" (6 Months - Years) 26 Ownership of Nursing Facilities Over 15,000 freestanding nursing home facilities in 2012. 1.7 million licensed beds. 85.0% average occupancy (93% 1994) 26.6% of NH beds are in large nursing home chains. 50 - 66% of nursing home revenue is from Medicaid 27 . 28 Adapted from Kaiser State Health Facts, 2007 Resident ADL Limitations in Nursing Facilities 100% 95% 87% 80% 79% 74% 60% 50% 40% 20% 0% Bathing 29 Dressing Toileting Transferring Eating Adapted from Cowles, CM, Nursing Home Statistical Yearbook, 2001, Cowles Research Group, Montgomery MD 30 42 02 Overview Nursing Facility Residents Dependency Status Home Health Care 3 ADLs 5% 2 ADLs 8% 1 ADLs 7% Medicare Covered Services 0 ADLs 5% 4 ADLs 23% 5 ADLs 52% Adapted from Cowles, CM, Nursing Home Statistical Yearbook, 2001, Cowles Research Group, Montgomery MD 31 32 Adult Day Care/Adult Day Health Care Home Health Care: Payer Mix Private Insurance 18.3% Part time skilled nursing, PT,OT, HH aides 80% of DME. Must be home bound (“out to physician’s office only.”) Physician determines need & sets up plan of care. Agency accepts Medicare payment as payment in full. Both licensed community based programs that provide health, social, and supportive services. Both programs typically provide meals, activities, and supervision by professionals. Adult Day Care Attendees have a personal care plan. Medicaid 24.8% Medicare 32.3% Patient (Self-pay) 16.5% Other Public 5.5% Source: CMS, Office of the Actuary, National Health Statistics Group Physical and cognitive impairment Socially isolated Persons needing assistance with personal care Provides a safe and caring setting for adults who cannot be left at home alone. 34 PACE Adult Day Health Care 33 Adult Day Care/Adult Day Health Care In addition to features of adult day care, Offers medical services, like rehabilitation, therapy, nursing care and special nutrition in addition to social and support services. PACE (Program for All Inclusive Care of the Elderly) Eligibility Criteria 55 years of age or older Able to live safely in the community at the time of enrollment Live in a PACE service area Certified as requiring nursing home level of care Dependent in ADLs Medical needs (DM, CHF, Dementia) 35 43 02 Overview PACE PACE Services Include Funding (unique arrangement with the federal and state governments) Medicare Medicaid and Private pay options Coverage includes: All of the Medicare (Acute care, Subacute care, SNF, skilled home care) as well as Medicaid ( NF, medical and social respite) Typically includes: Most Assisted Living Care is Privately Financed Assisted Living Adult day health care Transportation Home health and personal care services PT, OT, ST SNF & NF services Inpatient and outpatient services All Medicaid and Medicare covered services provided DME, laboratory services, medical supplies, all prescription medications Medical care provided by physician and IDT of healthcare professionals familiar with history, needs and preferences of each participant. 3 meals a day 24 hour security help with ADLs reminders about medicines assistance with appointments/transportation “Non medical environment”, but what about the medical stuff? 39 Care is self directed. Facilities are not responsible for coordinating medical care. No requirement for a medical director, but individual facilities are experimenting. 40 Hospice: Growth in 2009 to 1.2M! Assisted Living Source: 2006 Overview of Assisted Living 41 Source: National Hospice and Palliative Care Organization at www.nhpco.org/public/articles/provider graph 42 44 02 Overview Hospice: Qualifying Diagnoses Hospice: Ownership Non-For-Profit 49% For-Profit 47% Government 1992 2007 All Cancers 75.6% 41.3% All Non-cancers* 24.4% 58.7% *Major non-cancer diagnoses included CHF, COPD, stroke, Alzheimer’s Disease 4% NHPCO Facts & Figures, Oct. 2008 43 Hospice: OIG Report 2011 44 Hospice: OIG Report 31% of hospice beneficiaries lived in nursing facilities. Over 50% had at least 25% of their beneficiaries in nursing facilities and 19% had more than half. Close to 8% of all hospices had twothirds or more of their beneficiaries residing in nursing facilities (high percentage), total of 263 hospices. Of h 2% f fi d Medicare paid $3,182 more per beneficiary served by the high percentage hospices than hospices in general. Medium number of days for the high percentage hospices was 3 weeks longer than medium days for a typical hospice beneficiary, 52 days vs. 31. 28% of beneficiaries in high percentage hospices received more than 6 months The Nation’s Health Dollar, CY 2009 Part III: Payors for Long Term Care Medicare, Medicaid, and SCHIP account for one-third of national health spending. 1 Social Security Medicare Medicaid Patients/Families Medicaid and SCHIP 15% Other Public 12% 2 Other Private 6% CMS Programs 33% Private Insurance 34% Medicare 17% Out-of-pocket 15% Total National Health Spending = $2.5 Trillion 1 Other public includes programs such as workers’ compensation, public health activity, Department of Defense, Department of Veterans Affairs, Indian Health Service, and State and local hospital subsidies and school health. 2 Other private includes industrial in-plant, privately funded construction, and non-patient revenues, including philanthropy. 47 Note: Numbers shown may not sum due to rounding. Source: CMS, Office of the Actuary, National Health Statistics Group. 48 45 02 Overview The Nation’s Health Dollar, CY 2009 It All Began Here in 1935! Hospital and physician spending accounts or more than half of all health spending. Other Spending 24% Hospital Care 32% Program Administration and Net Cost 6% Prescription Drugs 9% Nursing Home Care 7% Physician and Clinical Services 22% Total Health Spending = $2.5 Trillion Source: CMS, Office of the Actuary, National Health Statistics Group. 49 50 SSA Amendments of 1965, enacted Title XVIII, Medicare Act Social Security Act (SSA) New Deal Program of 1935. Eliminating poor houses. Social Security for retired workers. Old Age Assistance for aged poor. Linked to prior earning and length of work history. Beginning of the Welfare State. Medicare Part B premiums are now deducted from social security checks. 51 52 Medicare: Title XVIII Medicare Health insurance program for all people 65 or older who are eligible for Social Security. The culmination of thirty years of national discussion and debate on national health care. Compromise bill preferred over a national health care plan. Part A Provides a defined amount of hospital care for everyone. Part B provides physician and other medical services for those who pay a monthly fee. President Johnson signing the legislation in 1965. “Social Security Amendments of 1965”. Optional program Part D provides prescription drug coverage. 53 Includes limited long term care benefits. Funded by payroll taxes with a trust fund. Optional program 54 46 02 Overview Medicare Spending Overall Medicare spending grew from $3.3 billion in 1967 to nearly $294 billion in 2004 and $502 billion in 2009. $350 294 $300 $241 $250 Dollars in Billions $211 $200 $132 $150 $81 $100 $50 $50 $3 $9 1967 1972 $23 $0 1977 1982 1987 1992 1997 2004 Fiscal Year Note: Overall spending includes benefit dollars, administrative costs, and program integrity costs. Represents Federal spending only. Source: CMS, Office of the Actuary. 55 56 Medicaid: Title XIX Medicaid Also passed in 1965 as an amendment to the Social Security Act. Social assistance program of health insurance for the Aged, Blind, and Disabled poor (the “ABD” population). 57 Jointly funded by the federal and state governments. Federal share is 50-83% Balance is state and local Based on per capita income Three types of critical health protection: Health insurance for low-income families and people with disability. Long term care for older Americans and individuals with disabilities. Supplemental coverage for low-income Medicare beneficiaries for services not covered by Medicare (medications) and Medicare premiums, deductibles, and cost sharing. 58 Part IV: Healthcare Industry Trends Medicaid Increasing: Stakeholder involvement Financial Stressors / Controlling Costs Survey and Regulatory Reform Workforce Shortage 59 60 47 02 Overview Involvement of Stakeholders Stakeholders Payors Patients and Families Medicare, Medicaid Insurance Companies Managed Care Regulatory Agencies Determines direction of revenue flow Determines standards Customers Varies Expectations Access to Information Quality Indicators Who are the stakeholders? 61 Financial Stressors Advocate Organizations 62 American Medical Directors Association (AMDA) American Healthcare Association (AHCA) American Association of Homes and Services for the Aging (AAHSA) National Association of Directors of Nursing Administration (NADONA) American Society of Consultant Pharmacists (ASCP) National Citizens’ Coalition for Nursing Home Reform (NCCNHR) 63 Occupancy Trends Occupancy Rates Down Reimbursement Changes Liability Costs Up Competition from Assisted Living Number of Nursing Facilities Median Occupancy Rate 17,500 96% 94% 93% 17,250 92% 92% 91% 91% 17,259 17,121 17,000 90% 90% 17,083 16,886 16,750 16,706 87% 87% 88% 86% 16,675 16,500 86% 86% 85% 16,389 16,250 84% 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 16,000 1995 1996 1997 1998 1999 2000 2001 Source: HCFA-OSCAR Form 1539 and Form 672:F78 OSCAR = Online Survey, Certification, and Responding 65 Adapted from Cowles, CM, Nursing Home Statistical Yearbook, 2001, Cowles Research Group, Montgomery MD 66 48 02 Overview Average Deficiencies Going Up! Now 8 in 2009 Increasing Liability Costs Annual Number of Claims per 1,000 Beds Average Deficiencies 5.50 5.1 5.00 5.00 5.5 5.3 4.50 4.00 4.00 4.00 3.50 3.00 3.00 3.00 2.50 2.00 1997 Office of the CMS Actuary, 2003 67 1998 2002 1999 2003 1999 2000 2001 2002 2003 2004 Source: HCFA-OSCAR Percent Deficiency-Free Down! 1997 2001 1998 68 Regulatory Reform Not Whether, But How! 2000 2004 Attitude Shift: From Punitive to Collaborative 25.00 22.30 Focused on Quality Improvement 20.50 20.00 17.70 15.00 Fair, Fast, Final Dispute Resolution Process (IDR) 15.00 13.50 Revise Policies Related to Mandatory Termination 12.1 10.5 10.00 1997 1998 1999 2000 2001 Source: HCFA-OSCAR 2002 2003 10 2004 Redirect Poor Performing Chains Policy “The beatings will stop when attitudes improve”. 69 Workforce Issues 70 Long Term Care: Future Caregiver Supply Salary Competition Job Demands Regulatory Climate 71 More older patients, not enough beds. More older patients, not enough caregivers. More older patients, not enough resources to pay for care. Less working persons per beneficiary to pay for Medicare, Social Security of the elderly. 72 49 02 Overview Part V: Using Your CMD Skills Long Term Care: Future Affordable Care Act of 2010 Accountable Care Organizations Bundling of Services Value Based Purchasing Electronic Medical Records E-prescribing Medical Homes Diagnosing and Treating Your Nursing Facility! 74 Diagnosing Your Nursing Facility (NF) Diagnosing Your NF Patient Complaints Practitioner vs. Nursing Home Facility concerns Medical Director Core Skills / Knowledge Regulations Financial Issues Medical Care Delivery Systems Medical Director Contract Healthcare Ethics Governance Core Skills History Evaluation Diagnosis Intervention History Sources of Information Board ED Administrator Nurse Practitioner/CNS/Physician Assistant DON Unit Manager Nursing Nursing Assistants Make it a routine. Weekly/Monthly attendance at QA, Medicare A, QOL, Falls, Wound Rounds (Committees) Follow up builds your power base and influence. 50 02 Overview Evaluation Evaluation OSCAR Data MDS Data QI/QM reports Incident Reports In House QA data- Falls, Wounds, Wt Loss Infections Lab Radiology Pharmacy Diagnosis PROCESS Sequence of tasks aimed at accomplishing a goal. Produce data which can be analyzed. Diagnosis First: Your current processes are perfectly designed to get the results they are already getting and designed to get, With it's corollary: Insanity is doing things the way you have always done them while expecting different results. Diagnosis- PDCA 85/15 Process Rule Individuals have direct control over only 15% of their work problems. The other 85% are controlled by the process in their work environment. Review Current Policies / Procedures Employee Health Infection Control Resident Rights Medical Record Coordination of Medical Care Risk Management Plan START Act Do Deming 4% - 96% Check 51 02 Overview Complete MDS Admission Nursing Assessment Does Falls RAP Trigger? Yes No Is there any other reason to believe patient is at high risk for falls? Identify modifiable intrinsic or extrinsic risk factors Yes No Establish Care Plan Routine Precautions Fishbone Diagram CNA assistance with meals Inadequate training Hospice Ortho Rehab Example Facility Weight Loss > 5 lbs and Below IBW Short staffed Type of Patient Obese patient on diet High toileting needs Holiday call-offs Lack of interest Wages not competitive 30% Don’t understand importance Weight Loss New Dietician Mean 22% UCL 18% LCL 10% Ju n96 Au g9 O 6 ct -9 6 D ec -9 6 Fe b97 A pr -9 7 Ju n97 A ug -9 O 7 ct97 De c97 Fe b98 Poor presentation Wrong Temperature Monotonous Menu Dietary Staffing 26% 14% Wages not competitive Holiday call-offs 34% Food Not Appetizing Control Chart 87 Weight Loss > 5lbs and Resident Outside Ideal Weight Intervention Changed timing 0.40 Cycle 1 0.35 0.30 Process Improvement Cycle 2 0.25 Cycle 3 0.20 0.15 2 oz with meds 0.10 Phase 1 – Stabilization Phase 2 – Active improvement Phase 3 - Monitoring Cycle 4 Other supps Flavor variety 0.05 UCL= 0.304 D ec-0 3 Ju n - 0 3 D ec-0 2 Ju n - 0 2 D ec-0 1 Mean= 0.197 Ju n - 0 1 D ec-0 0 Ju n - 0 0 D ec-9 9 Ju n - 9 9 D ec-9 8 Ju n - 9 8 D ec-9 7 Ju n - 9 7 D ec-9 6 Ju n - 9 6 D ec-9 5 Ju n - 9 5 D ec-9 4 Ju n - 9 4 D ec-9 3 Ju n - 9 3 D ec-9 2 Ju n - 9 2 D ec-9 1 Ju n - 9 1 D ec-9 0 Rate 89 LCL= 0.090 90 52 02 Overview Intervention The Big Picture Systems Theory/Problem Solving Leadership Personality Profiles Influencing Employee Behaviors Working with Families System Process People • Group of related interdependent processes working together to achieve a common goal • Made up of a culture, structure and boundary • Sequence of tasks aimed at accomplishing a goal • Produce data which can be analyzed • Have beliefs, values, interests, needs • Have roles which are made up of functions and tasks Solutions Will Be Needed! Leadership Know what the right thing is And do it! Changing the paradigm of long term care. AMDA is here to do this! You are here to do this! Welcome to the CMD course! 93 53 03 Regulatory Environment Learning Objectives 03 Regulatory Environment Core Curriculum on Medical Direction List the long term care regulatory agencies and describe their process of developing and enforcing regulations Describe the survey process, the types of surveys, and responses to deficiencies Delineate the ways in which the medical director may assist the facility in complying with local, state, and federal regulations 2 1 Learning Objectives Outline for This Session: Define the medical director’s tasks in a survey visit Describe the role of the medical director and the associated investigative protocol for F501. Describe the special emphasis and regulations regarding medication use in long-term care 3 History of regulation. The regulation-making process. Types of surveys and the survey process. Response to deficiencies. Medical director’s tasks in the survey process. Regulations regarding the F-501 tag, Medical Director Requirement in Nursing Homes. Breakout session- Survey deficiency case analyses. Regulations regarding medication use and monitoring. Case studies in medication monitoring. 4 History: Why the Federal Government is Involved in LTC History: Why the Federal Government is Involved in LTC “Old Folks Homes”: prior to 1965 Institute of Medicine 1965: Medicare and Medicaid study – begun in 1983 report – issued in1986 Recommended SNF regulations – 1974 ICF regulations - 1976 OBRA ‘87 - 1987 implementation began in 1990 enforcement expanded in 1995 revised State Operations Manual (SOM) – 1999 5 re-design of the survey process use of a data driven approach 6 54 03 Regulatory Environment Purpose and Intent of NH Regulations History: Why the Federal Government is Involved in LTC Senate Committee on Aging hearings July 1998 Quality of care issues Survey process questioned Ensure that facilities meet minimum requirements for participation in payment programs -Medicare/Medicaid Thereby, hopefully, to ensure that residents reach the Clinton initiatives in response to above Off-hour surveys, remove predictability Focus on dehydration, malnutrition, pressure sores Highest practicable physical Mental Psychosocial well-being 7 8 Health Care of the Elderly is a Team Effort Purpose and Intent of NH Regulations Promote a coordinated interdisciplinary approach to: Optimize function Minimize preventable negative outcomes such as serious injury due to correctable causes of falling Manage complex problems such as impaired behavior appropriately and with regard for risks associated with interventions such as psychoactive medications and restraints The Goal: To Achieve: Synergy The action of two or more that achieves an effect which each is individually incapable of doing. Collaboration To work together in a joint effort in a spirit of cooperation. In other words, promote the applications of effective geriatrics approaches to care 9 10 Underlying Realities Related to Regulations Reference Sources for Regulations Many negative things happen to frail, elderly individuals. Many negative outcomes are unavoidable, while others result at least partially from process failures. Medicare and Medicaid beneficiaries receive many treatments, including medications. These treatments do varying degrees of good and harm. It is reasonable to ask for evidence that efforts were made to minimize the known risks and maximize the benefits. Federal publication State publication Similar to Federal, splitting proposed and final Commercial/trade publications 11 Federal Register: proposed regulations Code of Federal Regulations (CFR): final regulations For example, AHCA Administrator AMDA Web Site Links 12 55 03 Regulatory Environment Process: How Are Regulations Born? State Regulations Vary from state to state. May be stricter, but not more lenient that Federal regulations. All states conduct the OBRA survey on behalf of the Federal Government. Separate Federal surveys, including “lookbehind.” Statutory authorization Development Implementation 13 14 Process: Evolution of OBRA Statutory Authorization Process: Development CMS DHHS Cycle: Fed Reg. OMB Omnibus Budget Reconciliation Act (OBRA) of 1987, Public Law 100-203, included The Nursing Home Reform Amendments. Signed into law by Ronald Reagan. 1. Preamble and Rule 2. Final Rule Public Comment 15 16 Process: Evolution of OBRA Implementation Process: Evolution of OBRA Development CMS DHHS October 1990 - Federal and State surveyors start using interpretive guidelines for survey process. July 1995 - New enforcement procedures. July 1999 - Revised State Operations Manual (SOM), containing procedures for conducting the survey, enforcement, and other related processes. Cycle: Fed Reg. OMB 1 2 1. Preamble and Rule 2. Final Rule 3. Final Final Rule 3 Public Comment 17 18 56 03 Regulatory Environment Jargon DHHS OMB Federal Register Medicare - Title 18 Part A Part B Medicaid - Title 19 Jargon CMS IOM MDS Triggers RAPS OBRA ’87 OSCAR Key Players CMS NCCNHR AHCA AAHSA AARP AMDA 19 20 Jargon Survey Types Enforcement Scope (wide spread) Severity Remedies plan of correction civil money penalties denial of payment management termination Dispute mediation; appeal Standard Extended survey If significant issues in the following areas Quality of life Quality of care Resident behaviors Must be done within 14 days of end of standard survey. 21 22 Survey Types Standard Survey Abbreviated standard Complaint Change in management Partial extended Done if substandard care is found in abbreviated survey. Post-survey revisit Monitor implementation of plan of correction. 23 All nursing facilities and skilled nursing facilities that are certified to participate in the Federal Medicare Program and/or the State Medicaid Program must be surveyed at least annually to determine whether the facilities are in compliance with the Requirements of Participation. 24 57 03 Regulatory Environment Standard Survey Standard Survey Requirements of Participation Requirements of Participation 483.12 Requirements for Admission, Transfer and Discharge 483.13 Resident Behaviors 483.15 Quality of Life 483.20 Resident Assessment 483.25 Quality of Care 483.30 483.35 483.40 483.55 483.60 483.65 483.70 483.75 Nursing services Dietary services Physician services Dental services Pharmacy services Infection control Physical environment Administration 25 26 Standard Survey Standard Survey Requirements of Participation Requirements of Participation Each of these requirements have one or more associated “F-tags.” Lack of compliance with a requirement of participation. 483.40 Physician Services 483.40(c)(1): F Tag 387 Deficiency: The Resident must be seen by a physician at least once every 30 days for the first 90 days after admission, and at least once every 60 days thereafter. 483.20 Resident Assessment 483.20(k)(3)(I): F Tag 281 The services provided or arranged by the facility must meet professional standards of quality. 27 The Survey Process Overview OBRA - Enforcement Possibly: To identify “poor performers.” 28 Timing of survey Notification Must be unannounced Usually annual Timing- generally 8:00AM - 6:00PM CMS requires that at least 10% of surveys must begin on the weekend or in the evening or early morning hours. But essentially: Looking for substantial compliance. Remedies for deficiencies based on the scope and severity of what is found. 29 30 58 03 Regulatory Environment The Survey Process Overview The Survey Process Overview Surveyors are required to conduct the survey in accordance with the regulations and interpretive guidance set forth in the State Operations Manual (SOM). The facility must prepare a Plan of Correction (POC) for each violation and notify the State Survey Agency of a date by which the facility will be in compliance. If deficiencies are found, the survey team will issue a Statement of Deficiencies: The State Survey Agency or CMS may conduct a post-survey revisit to assure adherence to the POC. For minor deficiencies, only a letter from the facility confirming compliance is necessary. Summarizes findings. Identifies failure to comply with regulatory provisions. Categorizes the seriousness of the violations based 31 on a matrix created by the CMS. 32 The Traditional Survey Process The QIS Survey Process Elements of the Traditional Standard Survey Pre-visit survey (Task 1) Entrance Conference & On-Site Preparation (Task 2) Initial Tour (Task 3) Sample Selection (Task 4) Information Gathering (Task 5) Determination of Compliance (Task 6) Exit Conference (Task 7) QIS Survey Development began 1994 University of Colorado States involved 2005 CT, OH, FL, LA, KS 2006 CA 2008 NM, MN, NC 2009 WV, MD, WA 33 34 The QIS Survey Process The QIS Survey Process Automated During Stage 1 residents are randomly selected. QIS survey designed to: Interviews with Residents (83% are interviewable) that a proscribed. Interview with staff, families. Observations of those non interviewable residents. 35 Improve consistency and accuracy of QOC and QOL problem identification. Enable timely and effective feedback on the survey process. Systematically review requirements and objectively investigate all triggered regulatory areas. 36 59 03 Regulatory Environment The QIS Survey Process The QIS Survey Process QIS survey designed to: Elements of the QIS Survey Provides tools for continuous quality improvement. Enhance documentation. Focus survey resources. 37 Differences Between Traditional and QIS Survey Process Information requested: QI/QM report Roster sample matrix Tour Gather information about concerns that have been preselected, new concerns, and other candidates for the phase 1 sample. Are preselected residents still present. Information requested: Alphabetical list of all residents and their room numbers. List of new admissions and discharges over the last 30 days. Tour Initial review to gain information about the resident population, staff and facility layout. Not information gathering. Traditional Sample selection Residents selected offsite based on facility’s QI’s of concern. Determine whether any preselected concerns should be substituted based on review of Roster/Sample Matrix and findings from the tour. Determine Phase 1 sample residents who are interviewable. 39 Differences Between Traditional and QIS Process Traditional Survey Structure Phase I involves both comprehensive and focused reviews. Phase II focused and closed reviews. QIS Survey Structure Stage I involves a preliminary investigation of all regulatory areas in Admissions, Census, and Surveyor-initiated samples. Stage II involves further investigation of triggered Care areas in Stage II sample chosen based on Stage I findings. 41 38 Differences Between Traditional and QIS Survey Process QIS Traditional Task 1: Off site preparation Task 2: Onsite preparatory activities and entrance conference Task 3: Initial tour Task 4: Stage 1 survey tasks Task 5: Non-staged survey tasks Task 6: Transition from stage 1 to stage 2 Task 7: Stage II survey tasks Task 8: Analysis and decision making Task 9: Exit conference QIS Sample selection 4 samples selected by QIS DCT: MDS offsite sample-residents with an MDS within 180 days. Random Admission sample-30 residents admitted more than 30 days prior to survey. Random Census sample-40 residents currently in facility selected through offsite and onsite activities. Surveyor-initiated sample-resident’s selected at surveyor’s discretion. 40 The QIS Survey Process www.Nursinghomequality.com 42 60 03 Regulatory Environment The Traditional Survey Process Pre-visit Preparation (Task 1) The Survey Process Contact ombudsman Review 2567, previous level A citations Review OSCAR Report 3 (Hx facility profile) Review OSCAR Report 4 (Full facility profile) Review results of interim complaint investigations Any outstanding complaints PASSAR reports on MI/MR Review waivers/variances MDS assessments Quality Monitor/Indicators Report Quality Indicator/Quality Measure Reports Facility characteristics Facility level summary Resident level summary Resident census and conditions Resident roster/sample matrix 43 44 Quality Measures/Indicators 13 Domains/31 Indicators + 3 Post Acute Indicators For example, the Accidents “domain” has two “indicators” Accidents 1) Incidence of new fractures 2) Prevalence of falls This will report for any time frame selected the number of residents with new fractures (facility acquired) and with falls, calculate the % rate, compare that rate with state and national rates, and provide a percentile rank relative to all facilities in your state. 45 46 The QIS Survey Process Pre-visit Preparation (Task 1) Similar to Traditional process tasks but: Surveyors do not review QI/QM data and OSCAR 4 reports or preselect residents for review. MDS is loaded offsite into surveyors computers and are used to calculate QCI’s and randomly select residents for Stage I. QCI=Quality of Care Indicators 47 3.1 – 3.5 48 61 03 Regulatory Environment The Survey Process The Survey Process Entrance Conference and On-Site Preparation (Task 2) Entrance Conference and On-Site Preparation (Task 2) Team coordinator informs administrator of survey and introduces team. Team members proceed to initial tour. Survey team Team coordinator Has at least a nurse and dietician. A pharmacist may be involved. Depending on facility size and issues, more nurses or other disciplines. Requests copy of working schedules for licensed staff and registered nursing staff by the end of the tour. Informs facility team will communicate with them and give facility the opportunity to clarify issues brought to facility’s attention. Give administrator OSCAR reports 3 and 4. 49 50 The Survey Process The Survey Process Entrance Conference and On-Site Preparation (Task 2) Entrance Conference and On-Site Preparation (Task 2) Team coordinator Asks administrator about any special features of the facility. Requests time to meet with residents/families without the facility staff present. Request additional documents within 24 hours of Entrance Conference. Within 1 hour of conclusion of Entrance Conference the facility must provide: List of key personnel. Copy of written information given out regarding Resident Rights. Meal times, dining locations, copies of all menus to be used during the survey. Medication pass times. Copy of the facility layout, AND…. 51 52 The Survey Process The Survey Process Entrance Conference and On-Site Preparation (Task 2) Entrance Conference and On-Site Preparation (Task 2) Within 1 hour of conclusion of Entrance Conference the facility must provide: Copy of the facility admission contracts for all residents. Copy of policies and procedures to prohibit and investigate allegations of abuse. Evidence that facility routinely monitors accidents and other incidents, and its system to minimize them. 53 Within 1 hour of conclusion of Entrance Conference the facility must provide: Current resident activity calendar. Names and ages of residents 55 and under. Names of residents who communicate with non-oral devices, sign language, or speak language other than dominant language in the facility. 54 62 03 Regulatory Environment The Survey Process The Survey Process Sample Selection (Task 4) Initial Tour (Task 3) Brief visit to the kitchen early on Day 1. Confirm or invalidate “pre-selected concerns.” Procedures dictate sample selection based on the resident census. Types of reviews include: Comprehensive reviews Focused reviews Closed record review Resident family interview W.H.P. group (unintended weight loss, hydration, or pressure sores) 55 56 The Survey Process The Survey Process Sample Selection (Task 4) Approximately 60% of the sample was preselected prior to entry. Statutory requirement for sample to be casemix stratified. Current (2009) “hot button” issues are F 329 Medication Management F 309 Pain Management If any resident flags under W.H.P. Quality Indicators, at least 50% of sample must include residents with these conditions. 57 58 Investigative Protocols Resident Sample Selection For selected conditions spells out specific criteria and protocol: Note, W, H, P Adverse Drug Reactions (ADR) and Inappropriate Medications (2006) Pressure Sore/Ulcer (2004) Hydration (2004) Unintentional Weight Loss (2007) Medical Director (2005) Urinary Incontinence and foley catheters (2005) Pain Management (2009) 60 63 03 Regulatory Environment The Survey Process The Survey Process Information Gathering (Task 5) Determination of Compliance: Task 6 5A General observations 5B Kitchen/Food Service 5C Resident Review 5D Quality of Life Assessment 5E Medication Pass Observation 5F Quality Assessment and Assurance Program 5G Abuse Prohibition Review Assesses problems based on Scope - How wide spread is it Severity - Degree of harm And derives a grid score by letter (A through L) for each deficiency. Higher letters generally indicate more trouble for the facility. 61 62 OBRA – Enforcement OBRA – Enforcement Deficiency Scope Levels Deficiency Severity Levels Scope can be: LEVEL Isolated Pattern Widespread EXAMPLE No actual harm with potential for minimal harm No actual harm with potential for more than minimal harm, no immediate jeopardy Actual harm, no immediate jeopardy salt on NAS tray; failing to follow a physician order for a routine lab test poor sanitation; omitted dose of important, but not critical, medication puree not smooth, resident chokes; failure to turn & position, then pressure ulcer poor refrigeration of food; toxic item in food; no program to prevent/heal ulcers Immediate jeopardy to reident’s health or safety 63 64 G: puree not smooth, resident chokes OBRA - Enforcement OBRA - Enforcement Severity IMMEDIATE JEOPARDY POC REQ: CAT 3 OPT: CAT 1 OPT CAT 2 POC POC J Sub. REQ: CAT 3 CAT 1 QOC OPT: OPT CAT 2 POC Act Harm; REQ: CAT 2 no immediate OPT: CAT 1 G jeopardy No Act Harm; POC REQ: CAT 1 >Minimal harmOPT: CAT 2 D no imm jeop POC No Act Harm; No COMMITMENT TO < Min Harm CORRECT POC REQ: CAT 2 OPT: CAT 1 POC REQ: CAT 1 OPT: CAT 2 A Subst. Compl. Isolated POC K Sub. REQ: CAT 3 CAT 1 QOC OPT: OPT CAT 2 H Sub. REQ: CAT 2 CAT 1 QOC OPT: OPT: TEMP L POC MGMNT POC REQ: CAT 2 OPT: CAT 1 E B Subst. Compl. Pattern POC I F Sub. QOC Sub. QOC Sub. QOC C IMMEDIATE JEOPARDY POC REQ: CAT 3 OPT: CAT 1 OPT CAT 2 Actual Harm; no immediate jeopardy No Act Harm; >Min harm no imm jeop No Act Harm; < Min Harm POC REQ: CAT 2 OPT: CAT 1 POC REQ: CAT 1 OPT: CAT 2 Sub. REQ: CAT 3 CAT 1 QOC OPT: OPT CAT 2 POC REQ: CAT 2 OPT: CAT 1 G POC REQ: CAT 1 OPT: CAT 2 D No POC COMMITMENT TO CORRECT Subst. Compl. A Subst. Compl. Widespread D: poor sanitation; soft-cooked egg Scope of the problem 65 POC POC J Isolated K Sub. REQ: CAT 3 CAT 1 QOC OPT: OPT CAT 2 H Sub. REQ: CAT 2 CAT 1 QOC OPT: OPT: TEMP L POC MGMNT POC REQ: CAT 2 OPT: CAT 1 E POC B Subst. Compl. Pattern POC I F Sub. QOC Sub. QOC Sub. QOC C Subst. Compl. Widespread 66 64 03 Regulatory Environment National Deficiency Data National Deficiency Data Trends in Deficiencies, 2005-2007 2008 “Trends in Nursing Home Deficiencies and Complaints” Reported by the CMS 2005 2006 2007 Percentage change 2005-2007 % of NF with deficiencies 91.5% 91.8% 91.9% 0.9% Avg # of deficiencies per NF 6.4 6.9 7.0 10.7% Total # deficiencies 95,624 102,487 104,665 9.5% Total #NF 15,046 14,954 14,872 -1.2% 67 68 Total Deficiencies by Class of Ownership 2007 National Deficiency Data 2007 Immediate Jeopardy J G 1.3% K 1.2% L 0.4% 14.1% H 1.1% I 0.1% D 82.4% E 62.8% F 21.2% No harm A 0.0% B 27.6% C 22.2% Isolated Patterned For Profit 7.6 Non-Profit 5.7 Government 6.3 Widespread 69 70 National Deficiency Data 2007 National Deficiency Data 2007 Percentage of NF receiving at least one deficiency by category Quality of care Resident assessment Quality of life 73.6 58.2 43.3 Summary 71 91% of NF were cited for deficiencies. Variation by state total number per facility. Greater number of for profit than not for profit are cited. 17% surveyed in 2007 cited for actual harm or immediate jeopardy. 3.6% were cited for substandard quality of care. 72 65 03 Regulatory Environment OBRA – Enforcement Substandard Care OBRA – Enforcement Substandard Care Substandard quality of care: Any survey deficiency in: That constitutes immediate jeopardy to resident health or safety; OR • 483.13 Resident Behavior and Facility Practices A pattern of or widespread actual harm that is not immediate jeopardy; OR 483.15 Quality of Life 483.24 Quality of Care Widespread potential for more than minimal harm that is not immediate jeopardy, with no actual harm 73 74 OBRA - Enforcement Immediate Jeopardy Severity IMMEDIATE JEOPARDY Act Harm; no immediate jeopardy No Act Harm; >Minimal harm no imm jeop No Act Harm; < Min Harm J Sub. QOC K G H D E Sub. QOC L Sub. QOC I F Sub. QOC The Guidelines also clarify that actual harm, as well as the potential for harm, to one or to more than one individual may constitute Immediate Jeopardy. Sub. QOC A B C Subst. Compl. Subst. Compl. Pattern Immediate Jeopardy: “A situation in which the provider’s noncompliance with one or more requirements of participation has caused, or is likely to cause, serious injury, harm, impairment, or death to a resident.” (See 42 CFR Part 489.3.) Sub. QOC Subst. Compl. Isolated Widespread Scope of the problem Ref: SOM: Appendix Q: Guidelines for determining Immediate Jeopardy (292 pages) 76 OBRA – Enforcement Substandard Care OBRA - Enforcement Severity If substandard care is cited, the State must notify: The state NHA licensing board. Attending physicians of residents. IMMEDIATE JEOPARDY POC REQ: CAT 3 OPT: CAT 1 OPT CAT 2 POC REQ: CAT 3 OPT: CAT 1 OPT CAT 2 POC REQ: CAT 3 OPT: CAT 1 OPT CAT 2 Act Harm; no immediate jeopardy No Act Harm; >Minimal harm no imm jeop No Act Harm; < Min Harm POC REQ: CAT 2 OPT: CAT 1 POC REQ: CAT 2 OPT: CAT 1 POC REQ: CAT 1 OPT: CAT 2 POC REQ: CAT 1 OPT: CAT 2 POC REQ: CAT 2 OPT: CAT 1 OPT: TEMP MGMNT POC REQ: CAT 2 OPT: CAT 1 No POC COMMITMENT TO CORRECT POC POC Subst. Compl. Subst. Compl. Isolated Pattern Subst. Compl. Widespread Scope of the problem 77 78 66 03 Regulatory Environment OBRA – Enforcement Categories of Remedies OBRA – Enforcement Categories of Remedies Category 1 Directed plan of correction. State monitoring and/or directed in-service training. Category 2 Denial of payment for new admissions. Denial of payment for ALL residents (imposed by CMS). And/or civil money penalties ($50 $3,000 per day; may be retroactive). 79 The Survey Process Exit Conference: Task 7 OBRA – Enforcement Categories of Remedies 80 Category 3 Temporary management Termination Optional civil money penalties ($3,050 - $10,000 per day; may be retroactive) 81 Deficiency and Plan of Correction OBRA – Enforcement Plan of Correction Defines the problem found in the survey deficiency. States how problem will be resolved. Methods Time frame States how facility will ensure that the deficiency does not/will not affect other residents. 82 Deficiency and reference regulation F- tag Justification for citation 83 Plan of Correction 84 67 03 Regulatory Environment OBRA – Enforcement “Informal Dispute Resolution” and Appeals “Informal Dispute Resolution” has relatively informal process; NO DELAY in enforcement. may have ONE hearing for appeal, using Federal or State process • Survey Reports Available on the Internet In an attempt to help customers select nursing homes based on quality, annual survey results and quality monitor/indicator data are available on line at: www.medicare.gov Look for: nursing home compare 85 86 Home Care and Hospice Quality Monitoring 3.6 – 3.11 87 Home Care and Hospice Home Care and Hospice Initiatives similar to those covering the nursing home industry are being carried over into other domains where Federal funds are involved: home care and hospice MDS, Minimal Data Set (NH) = UDS, Uniform Data Set (Home Care and Hospice) OSCAR (NH) = OASIS (Home Care/Hospice) 89 Outcome Assessment Information Set (Outcome and Assessment Information Set) “OASIS” Reported on-line O AS I S Full name is Medicare Home Health Care Quality Assurance and Improvement Demonstration Outcome and Assessment Information Set 90 68 03 Regulatory Environment Home Health Agency Quality Indicators Home Care and Hospice Outcome-based quality improvement. Modeled after the nursing home industry. Derived from a 5 year national research program to develop outcome measures for home care. Tested nationally and revised. 91 92 Home Health Compare Quality Indicators % who improve in walking or moving around % who improve in medication administration % who improve at getting in and out of bed % who improve getting to and from the toilet % who have less pain when moving % who improve in bathing % who get better at getting dressed % who stay the same at bathing % who had to be admitted to the hospital % who need urgent, unplanned care % whose confusion improves Improvement in Bathing Oasis Report Improvement in Toileting Improvement in Ambulation 93 94 The Medical Director’s Role F 501 Tag 3.12 – 3.14 95 69 03 Regulatory Environment Medical Directors & Federal Regulations Importance of the Medical Director Medicare regulations have required medical directors in SNFs since 1974. OBRA ’87 extended the requirement to nursing facilities (NFs). Federal regulations specify only two duties. Implementation of resident care policies. Coordination of medical care in the facility. 97 Importance of the Medical Director Medical directors accountable for the quality of care in LTC, but have little authority within facilities and over attending physician. Recommended vesting greater authority & responsibility in medical directors for medical services and require attending physicians and nurse practitioners to follow facility medical policies and procedures. 98 Revised Investigative Protocol for F-501 Tag (2005) “An Insider’s View: The Role of the Nursing Home Medical Director” (OIG Report, Feb 2003) Improving the Quality of Long-Term Care (Institute of Medicine report, 2001) Medical Directors are committed. Medical Directors value role more than administrators do. Inadequately defined regulatory role results in underutilization. No fundamental change in expectations or requirements but: Better defined the medical director’s importance. Clarifies the meaning of the original requirements. More details of essential functions and tasks. Standardized expectations for providers. Consistent with the core roles/functions identified by medical directors themselves. 99 100 Medical Directors & Interpretive Guidance Interpretive Guidance Medical Director’s Tasks Clarify meaning and implementations of basic federal regulations. Subject to periodic revision. Stakeholders can provide input. Original CMS Interpretive Guidelines defined seven functions for Medical Directors. 101 (1) Assuring that the facility is providing appropriate care as required. (2) Monitoring and ensuring implementation of resident care policies. (3) Providing oversight and supervision of physician services and medical care of the residents. 102 70 03 Regulatory Environment Medical Director’s Tasks Medical Director’s Tasks (4) Overseeing overall clinical care of residents to ensure to the extent possible that care is adequate. (5) Evaluating possibly inadequate medical care - including drug irregularities- identified or reported, evaluate and try to correct the problem. (6) If necessary, consult with resident and resident’s physician about care and treatment. (7) Assure the support of essential medical consultants as needed. 103 104 Medical Director’s Tasks Medical Director’s Tasks OBRA: Resident Care Policies (1 of 2) OBRA: Resident Care Policies (2 of 2) Admissions Treatment Discharge Infection control Use of restraints MD privileges & practices Non-MD staff Ancillary services Nursing Rehab services Dietary in res. care Emergency care Res. assessment & care planning Lab Radiology Pharmacy Use of medications Use and release of clinical information Overall quality of care “The Medical Director is responsible for ensuring that these care policies are implemented.” 105 106 Medical Director’s Tasks Medical Director’s Tasks The medical regimen must be part of an interdisciplinary care plan designed to: Try to achieve highest practicable physical, mental and social well-being. Preserve function Minimize injury/falls Minimize psychoactive meds/restraints Restraint use requires rigorous individualized clinical assessment, should be appropriate, and implemented only after considering other less risky alternatives. 107 Additional functional decline may be caused by inappropriate restraint use. Type, duration, indications, review, revision 108 71 03 Regulatory Environment Medical Director’s Tasks Proactive Measures to Ensure Year-Round Compliance Medical Director’s Tasks The resident’s drug regimen must be justifiable. Necessary, appropriate indications. Appropriate dose, duration and monitoring. Not duplicated unnecessarily. Monitoring of adverse affects. Attempts at drug/dose reduction, when indicated. Re-evaluate hiring and credentialing. Ensure that attending physicians provide a thorough, relevant, well-documented initial examination. Help physicians address consent-totreatment issues. 109 110 Medical Director’s Tasks Medical Director’s Tasks Proactive Measures to Ensure Year-Round Compliance Proactive Measures to Ensure Year-Round Compliance Ensure that physicians address the broad range of patient conditions, overall functional status, and quality-of-life issues in the proper context. Ensure that physicians develop documentation skills that reflect OBRA awareness – not OBRA obsession. Develop medical policies and procedures geared to effective geriatrics and compatible with OBRA guidelines. Help physicians in their relationships with residents and families. Actively help develop and implement an aggressive quality-assurance program. Participate in the survey process and in challenging questionable deficiencies. 111 112 Medical Director’s Tasks Survey Participation Pre-Survey Surveyor Investigative Protocol Medical Director (2005) Follow proactive measures listed above During the Survey Objective: “To ascertain whether the medical director, in collaboration with the facility, coordinates medical care and the implementation of resident care policies.” Introduce yourself if possible to surveyors in the building- business card? Be available to administration and to surveyors Show your presence and interaction with the staff and administration, at least some time during the survey Participate in exit conference if possible 113 Use this protocol when: The facility does not employ a licensed medical director, or the medical director is not currently licensed by the State. Concerns with the provision of resident care or medical care or Concerns with quality assurance related to the provision of medical or resident care. 114 72 03 Regulatory Environment Surveyor Investigative Protocol Medical Director Surveyor Investigative Protocol Medical Director During the survey process, the surveyor should attempt to communicate with the medical director about concerns related to: “When concerns are identified regarding the quality of care, quality of life, or protection and promotion of resident rights, the surveyor should evaluate the possibility of isolated or systemic failure of the provision of medical care in the facility.” Admission of residents whose care needs cannot be readily met by the facility. Access to or provision of physician or consultant services. Identification, assessment, or provision of services to meet resident needs. Capabilities and credentials of staff or other providers/contractors. Facilities success in honoring residents rights and enhancing personal dignity. Implementing and maintaining current standards of practice for resident care and quality of life. Effectiveness of the various committees responsible for overseeing resident care and quality of life. 115 “If the survey process identifies the facility’s lack of a functioning medical director or the lack of medical director involvement in implementing resident care policies and coordinating care, use the Medical Director Investigative Protocol.” 116 Surveyor Investigative Protocol Medical Director Surveyor Investigative Protocol Medical Director Facility/Medical Director responsibility for resident care policies. If the survey team identifies concerns related to the provision of resident care, investigate how the medical director, in coordination with the facility, provides input into the new development, review, revision, and oversight of the implementation of resident care policies. How was it determined that the policy reflected current standards of practice. If not available, interview the Medical Director about his/her involvement in implementing resident policies. 117 Surveyor Investigative Protocol Medical Director Coordination of medical care/physician leadership. Ensuring that provisions are in place for physician services 24 hours a day and in case of emergency. Ensuring that visits and orders are provided as required. Ensure that rules and procedures are established for ongoing coverage for physician services. Ensuring that practitioners, who are used to perform physician delegated tasks, act within the regulatory requirements and within their scope of practice as defined by State law; and ensure that they are under a physician’s supervision. Whether the facility identified problems related to care that needed her/his consultation, i.e. notification of a physician about resident changes. 119 Coordination of medical care/physician leadership. If the survey team discovers issues or concerns with resident care/medical care, determine how the facility obtains the medical director’s input in developing policies related to these issues and involvement in the coordination of medical care. Determine how the facility has involved the medical director in establishing and maintaining policies and procedures for credentialing physicians, nurse practitioners, physician assistants and other licensed or certified health care practitioners. Determine how the facility has involved the medical director in monitoring the provision of physician services. 118 Surveyor Investigative Protocol Medical Director Once the survey team has determined that non-compliance exists, the team will select the appropriate level of severity AND they must find a deficient practice at another tag . 120 73 03 Regulatory Environment Surveyor Investigative Protocol Medical Director Surveyor Investigative Protocol Medical Director Citation Examples The citation of a deficiency at F501, Medical Director, is a deficiency regarding the facility’s failure to comply with this regulation. The facility is in compliance if the medical director has assured that the facility has adopted and implemented relevant policies and procedures based on current standards and if the medical director has coordinated the provision of medical care and services in the facility. 121 Surveyor Investigative Protocol Medical Director Citation Examples Severity level 4 Must have a related care tag with actual harm and the Medical Director had knowledge of the issue. Timely antibiotic/medication delivery problem (widespread and known to the medical director) in a patient with pneumonia. Severity level 3 The surveyor must identify the relationship between the failed practices cited at other regulatory tags and the failure of the medical director to perform his/her functions. Stage 2 pressure sores in a facility with no pressure sore treatment protocols reviewed by the Medical Director. 122 Survey and F-Tags: Another “Hot Topic” Severity level 2 Must have a related care tag with no actual harm and the potential for more than minimal harm and the medical director had knowledge of the issue. Repeat lack of reporting of INR levels with the result that a patient’s anticoagulation profile is very high, but not bleeding. This is a facility wide problem and the medical director was aware. Severity level 1 There is a deficient facility practice but no negative resident outcome. The facility is searching for a new Medical Director. 123 Revision of the investigative protocol for F501 created anxiety for many medical directors. As it’s played out to date, this has not appeared to add additional work or legal liability (but the legal impact is perhaps not yet evident.) But there is always some issue rising to the top. 124 Medications in Long Term Care Special Concern and Additional Scrutiny 3.15 – 3.18 125 126 74 03 Regulatory Environment Medication Benefits and Risks Prevalence of Medications Medications can stabilize or improve outcome, quality of life, and function. Any medication can have adverse consequences. Potential to increase risk of adverse consequences. Study of 33,301 nursing facility residents in 2000. Average of 6.7 medications per individual. Twenty-seven (27) percent of residents on nine or more medications. Without adequate indications. Excessive dose Excessive duration Without adequate monitoring. Currently greater than 55% of all residents in long term care are receiving 1 or more mood altering drugs. 127 128 Medication Costs in the US: U.S. Total Drug Expenditures Total Drug Therapy Costs = Product Cost + Distribution Cost X Utilization U.S. Cost of Medication-Related Problems + Medication-Related Errors & Adverse Consequences $ 288 Billion Source: Parade Magazine, March 12, 2006 129 $ 111 Billion Medication (Ref: Ernst and Grizzle. J AM Pharm. Assoc., 2001) Management ADRs Increase With Number of Medications 130 Adverse Consequences: Evidence 131 $ 177 Billion 1.6 million US residents in nursing homes. Drug related injuries estimated to occur at a rate of 350,000 events per year. Thought that at least half may be preventable. 20,000 of these events may be fatal or life threatening and 80% of these may be preventable. Gurwitz JH et al. Arch Intern Med. 2002;162:1670-2 132 75 03 Regulatory Environment Adverse Consequences: Evidence History of Inappropriate Medication Regulation Review of 2 large academic LTC facilities 1229 beds in total 9 month record review 815 ADE which caused injury 188 deemed ‘Serious’ 33 ‘Life Threatening’ 4 ‘Fatal’ Most errors were in the prescribing and monitoring 10 ADE/month per 100 NH beds 60% of serious, life threatening and fatal were felt to be preventable Gurwitz JH et al. Am J Med. 2005;118:251-8 OBRA Regulations 1987 regulated the use of psychoactive medications in nursing homes. Beer’s Criteria HCFA (CMS) modified its regulations for nursing home residents in nursing homes in 1999. Revision of F Tag 329 (2006) 133 134 483.25 (I) - Unnecessary Drugs General Unnecessary Drugs: F329 Overview and Interpretive Guidelines Each resident’s drug regimen must be free from unnecessary drugs. An unnecessary drug is any drug when used: 135 483.25 (I) - Unnecessary Drugs Antipsychotics 1991 Nursing home residents over age 65 1997 Include all elderly regardless of setting 2003 Updated Based on a comprehensive assessment of a resident, the facility must ensure that: Residents who have not used antipsychotic drugs are not given these drugs unless antipsychotic drug therapy is necessary to treat a specific condition as diagnosed and documented in the clinical record; and Residents who use antipsychotic drugs receive gradual dose reductions, and behavioral interventions, unless clinically contraindicated, in an effort to discontinue these drugs. 137 In excessive doses (including duplicate therapy); or For excessive duration; or Without adequate monitoring; or Without adequate indications for its use; or In the presence of adverse consequences which indicate the dose should be reduced or discontinued; or Any combinations of the reasons above. 136 INTENT: (F329) 42 CFR 483.25(l) Each resident’s entire drug/medication regimen is managed and monitored to achieve certain goals 138 76 03 Regulatory Environment INTENT: (F329) 42 CFR 483.25(l) An individual receives only medications clinically necessary to treat assessed condition(s). Non-pharmacologic interventions considered and used instead of, or in addition to, medication when indicated. INTENT: (F329) 42 CFR 483.25(l) Medication or combination helps promote or maintain highest practicable physical, functional, and psychosocial well-being. Risks for adverse consequences or negative outcome(s) due to medication(s) are minimized. Appropriate doses for appropriate duration. For example, behavioral interventions for dementia-related behavioral symptoms. 139 140 INTENT: (F329) 42 CFR 483.25(l) If an individual experiences decline or newly emerging or worsening symptoms Change is recognized promptly. Medication regimen evaluated as potential contributing or causative factor. Changes made as appropriate. “Any symptom in an elderly patient should be considered a drug side effect until proved otherwise” Gurwitz, 1995 141 142 Purpose of F329 Surveyor Guidance Medication Management 143 Help surveyor determine whether the facility has a system for medication management that promotes key objectives regarding medications. 144 77 03 Regulatory Environment Guidance To Surveyors Key Considerations Guidance applies to all categories of medications including antipsychotic medications. Surveyor’s review of medication use not intended to constitute practice of medicine. Indications for use Dosage Duration Monitoring for effectiveness and adverse consequences Tapering / gradual dose reduction Preventing, identifying, and responding to adverse consequences. However, surveyors are expected to investigate basis for decisions and interventions. 145 146 What Can the Physician Do? Step One Proper assessment Make the right choices Monitor for efficacy Monitor for continued need Proper assessment Analyze the problem, do not treat the symptom. Avoid the prescribing cascade. History, physical exam and problem focused work up. 147 148 The “Cascade Effect” Step Two Right Drug Right Dose 149 Appropriate for the diagnosis. Appropriate for the older adult. Evaluate side effect profile and potential to cause drug drug interactions. Consider age related metabolism changes. Right Time Consistent with goals of care. 150 78 03 Regulatory Environment Step Three Monitor For effectiveness For continued need For side effects For drug drug interactions 151 152 Adverse Drug Withdrawal Events GDR: Antipsychotics Retrospective review of 175 VA NH residents. 94 ADWEs in 62 residents (35.4%) Cardiovascular and Psychoactive medications most common. Increased risk with number of diagnoses, number of medications and hospitalization during NH stay. Gerety M et al. J Am Geriatric Soc. 1993;41:1326-1332 Within the first year in which a resident is admitted on an antipsychotic medication or after the facility has initiated an antipsychotic medication, the facility must attempt a GDR in two separate quarters (with at least one month between the attempts), unless clinically contraindicated. After the first year, a GDR must be attempted annually, unless clinically contraindicated. For any individual who is receiving an antipsychotic medication to treat behavioral symptoms related to dementia, the GDR may be considered clinically contraindicated if: The resident’s target symptoms returned or worsened after the most recent attempt at a GDR within the facility; AND The physician has documented the clinical rationale for why any additional attempted dose reduction at that time would be likely to impair the resident’s function or increase distressed behavior. 153 154 GDR: Psychopharmacological Medications GDR: Antipsychotics For any individual who is receiving an antipsychotic medication to treat a psychiatric disorder other than behavioral symptoms related to dementia, the GDR may be considered clinically contraindicated if: The continued use is in accordance with relevant current standards of practice and the physician has documented the clinical rational for why an attempted dose reduction would be likely to impair the resident’s function or cause psychiatric instability by exacerbating an underlying psychiatric disorder; OR The resident’s target symptoms returned or worsened after the most recent attempt at a GDR within the facility AND the physician has documented the clinical rationale for why any additional attempted dose reduction at that time would be likely to impair the resident’s function or cause psychiatric instability by exacerbating an underlying medical or psychiatric disorder. 155 During the first year in which a resident is admitted on a psychopharmacological medication (other than an antipsychotic or a sedative/hypnotic), or after the facility has initiated such medication, the facility should attempt to taper the medication during at least two separate quarters (with at least one month between the attempts), unless clinically contraindicated. After the first year, a tapering should be attempted annually, unless clinically contraindicated. The tapering may be considered clinically contraindicated, if: The continued use is in accordance with relevant current standards of practice AND the physician has documented the clinical rationale for why any attempted dose reduction would be likely to impair the resident’s function or cause psychiatric instability by exacerbating an underlying medical or psychiatric disorder; OR The resident’s target symptoms returned or worsened after the most recent attempt at tapering the dose within the facility AND the physician has documented the clinical rationale for why any additional attempted dose reduction at that time would be likely to impair the resident’s function or cause psychiatric instability by exacerbating an underlying medical or psychiatric disorder. 156 79 03 Regulatory Environment Dementia Related Behaviors No FDA approved drug therapy for agitation. Impaired memory and processing can be mistaken for psychosis. Not all delusions are psychosis. Non-pharmacologic interventions are always first and second line choices. Pharmacotherapy should only be considered if nonpharmacologic interventions have failed and the symptoms are impairing function or causing danger to self or others. Pharmacotherapy in this setting is associated with an increase risk of falls, cognitive decline and functional 157 decline. Caring for the Ages, Nov 2007 Surveyor Expectations Monitoring for efficacy and ADR. Recognize change in condition as possible ADR. Performs Monthly Regimen Review Reports irregularities to MD and facility Facility and MD act on recommendation. 159 160 Implementation Of Resident Care Policies F Tag 501 and F Tag 329 Observation and record review. Interview resident, family, staff, clinicians, pharmacist. Review Medication regimen reviews. Pharmacist Non pharmacologic measure first if possible. Attempts at tapering if indicated and appropriate. Facility in collaboration with prescriber are Sources of Information Medications should have a clinical indication. 158 For thirty years, Medicare regulations have required medical directors in SNFs. OBRA ’87 extended the requirement to nursing facilities. Federal regulations specified two duties. Implementation of resident care policies. Coordination of medical care in the facility. 161 Medications have an indication clearly documented. Written physician orders for medications must include indication in order. Nursing will request indication if taking a verbal order. Significant resident change of condition such as weight loss, falls, functional decline and cognitive decline will be conveyed to consultant pharmacist on monthly basis. Facility has policy for appropriate follow up of medication regimen reviews generated by consultant pharmacist. 162 80 03 Regulatory Environment Coordination of Medical Care in the Facility Education of medical staff regarding principles of medication management and requirements of F329. Education of nursing home staff regarding monitoring for drug efficacy and potential side effects. Collaborating with consultant pharmacist regarding approach to MRR. Review MRR with nursing staff. OBRA and Medical Practice The practice of medicine is not regulated by CMS and OBRA… But the facility (and the Medical Director) are at risk if the attending physicians do not follow guidelines AND cannot justify significant deviations. Clinical judgment and practice must be based on medical standards and principles The literature contains ample evidence about medication-related problems in the elderly. ADRs are probably more common than recognized. 163 164 Case 1 OBRA and Medical Practice The answer to justification of disparities or discrepancies between guidelines and actual practice (translated into survey deficiencies) is clear, clinically relevant…. Mrs. B is an 88 year old female long term resident who you are visiting for your 60 day regulatory visit. Her major complaint is constipation. Staff reports 3 falls over the past 90 days. You note she has lost 20 pounds over the past 4 months. BP is 96/50, HR of 64. Her diagnoses include CAD, HTN, CHF, H/O PUD, Osteoporosis, and Overactive Bladder. Her medications include Metoprolol XL 100mg qd, Lisinopril 40mg qd, Digoxin 0.25mg qd, Furosemide 40mg qd, Diltiazem CD 240mg qd, Calcium Carbonate 500mg bid, Alendronate 70mg qweek, Omeprazole 40mg qd, Oxybutynin ER 10mg qd. What would you like to do next? 165 Case 2 Mr. C is a 78 year old male being treated for dementia related behaviors. He was started on Risperidone 0.5mg bid 4 months ago with moderate improvement in his symptoms. His family is grateful that he has improved. Your consultant pharmacist is asking for a GDR. What is your next step? What if this medication was being used to treat delusions and mania related to bipolar disorder? What if the medication was Sertraline and being used to treat depression? What if the medication was Galantamine? 167 166 Case 3 You are the new Medical Director of a 130 bed facility who has hired you to help improve care. Your review reveals that the facility is flagging in weight loss, late loss decline in ADLs and residents receiving 9 or more meds. Review of the consultant pharmacist report shows that 83% of the residents receive an antidepressant and 51% of the residents receive an antipsychotic medication. You are told that every admission receives a psychiatry consult. The psychiatrist is well regarded by the staff and all assessments and interventions are carefully documented. This physician has been overheard stating that it is difficult to be admitted to the nursing home and his medications help the resident to adjust. The MDS coordinator confides in you that there are frequent discrepancies between psychiatrist documentation and nursing home staff observation. What now? 168 81 03 Regulatory Environment Case 4 Case 5 Your consultant pharmacist reports that Dr. D is often ignoring his MRR, and when he does respond he always checks “Disagree” without offering any documentation or justification for his decision. Dr. D has been overheard by staff complaining about the over regulation of medicine and his distaste for others questioning his decisions. What now? Your DON and Administrator ask to meet with you because they are concerned about the new medication and pharmacy tags. It is there understanding that all new admissions need a MRR and anytime someone has a fall, loses weight or develops a pressure ulcer they need one as well. They are faxing over incident reports and notifications to the pharmacy but are being told the pharmacist cannot assess the resident every time they receive a fax. Your thoughts? 169 170 Case 6 You are reviewing prescribing habits with one of your attending physicians, who happens to have the highest number of medications per resident in the facility. When questioned, the physician states being frustrated about MRR recommendations for lipid and osteoporosis screenings and recommendations for starting cognitive enhancers in residents who have a diagnosis of dementia. “Once these recommendations are in the chart, I am concerned that if I do not agree with them, it will open me up for a law suit”. 3.19 – 3.22 Your thoughts? 171 172 82 04 Medical Information Management Learning Objectives Recognize the components and functions of a comprehensive medical record in long term care. Outline the tasks of the Medical Director that help ensure the integrity and clinical usefulness of the medical record. Discuss implementation of EMR in LTC setting. Describe legal and regulatory requirements, including HIPAA, that impact clinical documentation. 04 Medical Information Management Core Curriculum on Medical Direction 2 1 The Medical Record System has Multiple Functions and Users Chart organization Component location(s) Availability User friendliness Confidentiality 4.1 – 4.6 3 4 How Easy is it to Enter Clinical Data? How Good your System is Depends on Inputs and Outputs inputs outputs 5 Physician, nurse, therapist notes Nurse assistant ADL data MD orders Clinical monitoring, ie BS, BP’s 6 83 04 Medical Information Management How Easy is it to get the Data Out When and Where you Need it? LTC Records are more Comprehensive Than Those at any Other Site Advance directives/code status Labs/Xray reports Consultant reports Hospital records More regulations Longer length of stay More disciplines MDS and care plan 7 8 Medical Director’s Role is to Ensure Record…. The “Survey-Friendly” Medical Record Meets acceptable care standards. Is “physician-friendly.” Is timely, accessible, accurate. Fulfills regulatory requirements. Data is logically organized and consistent. Facility policies are followed. Entries are clear and legible. Addresses commonly posed questions by surveyors. Demonstrates effective communication with physician, resident and family. 9 10 Medico-legal/Regulatory Compliant Documentation Tips Medico-legal/Regulatory Compliant Documentation Tips Pressure Ulcers Identify risk factors (modifiable?) Implement preventive strategies Early recognition/Staging Interventions (consult?) Progress and prognosis Falls 11 Identify risk factors (modifiable?) Preventive strategies (?? restraint use) Realistic goals: e.g. reduce risk of injury Interventions—risk sharing Progress and prognosis 12 84 04 Medical Information Management Medico-legal/Regulatory Compliant Documentation Tips Medico-legal/Regulatory Compliant Documentation Tips Unintentional Weight Loss Antipsychotic Use Identify risk factors—avoidable or not? Implement preventive strategies Monitor progress Document discussion regarding alternate feeding method (i.e. tube feeding) Clear justification and evidence of reductions for: Use in geriatrics Use in dementia Two Antipsychotics Address informed consent, long term side effects, black box warning. 13 14 Medico-legal/Regulatory Compliant Documentation Tips Medico-legal/Regulatory Compliant Documentation Tips Family Conflict/Futility Describe dilemma and thought process. Be sensitive in reference to family members. Get second opinion. (? ethics consult) Interdisciplinary consistency in documentation is crucial. Medical Director can be second opinion/consult for clinically complex cases. Ensure appropriate documentation for sentinel events (dehydration, fecal impaction, low risk pressure ulcer). 15 16 The Medical Director has the Opportunity to: Provider Documentation: Medical Director Role Improve care and reduce litigation with simple improvements in the medical record. Participate with IDT in projects to improve usefulness of clinical record. 17 Staff education Oversight Supervision Role model 18 85 04 Medical Information Management Comprehensive SNF Resident Assessment Includes: After Initial Evaluation… Functional assessment Decision-making capacity At risk for: weight loss, falls, pressure ulcer, decline in status, etc. Goals for care/expectations Informed consent for psychotropics Regulatory visits Acute visits Annual evaluation Consider including: Functional status, problem list, current medications, social issues, family communication, intercurrent problems, prognosis 19 20 Regulatory Visits Regulatory Visits Everything that happened since last regulatory visit. Record should tell story. Subjective/objective findings. Summary Interim plan Long range plans, if changing. The more you do during the visit, the higher reimbursement( assuming medical necessity) 21 The Physician Annual Review is a Chance to Summarize and Update What has happened since last visit (nurse’s notes) Vitals, glucose, labs, consults Problem list Current medications, side effects Address ROS and SH (daily routine) Check skin 22 A Good Annual Exam: Review and update active problem list. Summarize current medications— appropriate monitoring/opportunities for discontinuation? Current functional status. 23 Summarizes overall goals for care. Lists specific plans related to findings from the entire review. Documents discussion of review, assessment and plan with resident or authorized representative. 24 86 04 Medical Information Management Might Want to Try: Might Want to Try: Medical Director consultation notes: Can bill if there is face to face contact with the patient, medical necessity and (preferred) request from attending. “At your service notes” high quality notes you write and send to the primary doctor, ideally to have them review and sign on key topics. Note templates for high risk situations 25 (eg Falls Pressure Ulcers Wt loss ) 26 Electronic Medical Record (EMR) What do you do If… Quality-council generated notes on inhouse pressure ulcers, antipsychotics, non-adherence. Templates for physicians (e.g. no-touch exam note, psychotropic justification, diabetic orders.) Physician is not visiting the facility for more than regulatory allowed intervals. Notes are illegible. Large in-house pressure ulcer and no documentation by the MD. 27 28 SNFs will be Following Along Very Soon The EMR in LTC—Beginning, not Quite Mature The Tide is Turning Toward EMR 29 Goal: Computerized record containing all data elements, accessible at all times to anyone with access privilege, integrates between systems. Reality: LTC facilities beginning cautious implementation of some components. 30 87 04 Medical Information Management AARA Offers Incentives for “Meaningful Use” There is no Turning Back In 2007, Minnesota mandated EMR in LTC. Standards being developed for LTC, studies show benefit. Federal and state mandates are likely coming to you soon. American Recovery and Reinvestment Act of 2009 invests millions of dollars in Medicare and Medicaid for meaningful use of certified IT products. 31 32 Meaningful Use Means… Last Point is Scary Use in a meaningful manner for selected functions. Allow electronic exchange of Health information (HIE) to improve quality. Use technology to submit to the Secretary real time clinical information on quality. Now the Secretary can analyze charts electronically to find the smallest errors. Surveyors may not just look at a sample, but can access all records, remotely, anytime. Many fear felony convictions for nursing home leadership related to quality of care. 33 34 Some are Moving Ahead, But Meaningful Use in SNF Carrot: Perhaps eligible for incentive payments from CMS. (Right now paying 10-30% of hospital implementation costs.) Stick: May keep from penalties. (Hospitals have to have meaningful use of certified EMR by 2014 or lose reimbursement.) 35 There is no certification yet for long term care and post acute facilities. Existing software choices are limited— especially in interfaces. No carrots and no sticks. 36 88 04 Medical Information Management Health Information Exchange (HIE) Who will Certify? Certification Commission for Healthcare Information Technology (CCHIT) Evaluates EMR Systems in 3 primary areas: functionality/security/interoperability Certification required to qualify eligible providers and hospitals for meaningful use stimulus program under ARRA. The idea is that everything can be integrated and accessible at your fingertips--hospitals, labs, X-ray, medical offices, pharmacy. National HIE is network of local and regional HIEs. 37 38 There are Challenges we are all Familiar With Staff turnover, skills, training. Money: high Medicaid population IT support—staffing, programs in short supply. Integration with providers inside and outside. Complex, highly regulated care. Return on Investment 39 Payback can come in as little as a year after implementing EMR. Saving staff time in documenting returns clinical time for resident care. Reducing waste and inefficiency and improving staff work life translates to lower turnover and higher satisfaction. Better documentation can mean more reimbursement. 40 Successful Adopters in LTC Said…. Care was better. Data was available, consistent and accurate. Reports helped track outcomes. Employees satisfied. Benefits outweighed costs. No-one wants to go back to paper. So let’s do it! 41 42 89 04 Medical Information Management Savage Gutkind EMR Implementation Model Most SNFs do not have a Full EMR, but have some Electronic Functions Financial and billing Staffing and timekeeping Generating face sheets and care plans MDS 0=manual 1=ADT and MDS 2=C.N.A. documentation 3= Order management Source: Savage-Gutkind EMR adoption model for LTC. 43 44 Savage Gutkind EMR Implementation Model 4=E-MAR and E-TAR 5= assessments 6=care planning 7=Clinical documentation 8=Ancillary integration 9=Decision support 10=Interoperable EMR Chief Information Officer Consortium EMR Cost Study for Long Term Care estimated EMR implementation costs at more than $250,000 per facility. 45 46 Resources Electronic Medical Records Cost Study http://www.leadingage.org/uploadedFiles/Co ntent/About/CAST/Resources/CIO_Consortiu m_EMR_CostStudy.pdf Agency of Health Care Research LTC EMR implementation study highlighted the challenges of EMR in long term care. As well as the significant benefits. 47 48 90 04 Medical Information Management Medical Information: EMR Medical Information: EMR Study: Cost, Staffing, Quality Impact of EMR in Nursing Homes. Example of EMR in LTC Facilities with EMR Higher Total/Nursing costs Similar Staffing ratios/retention Improved QIs/QM JAMDA 2010; 11: 485-493 49 50 EMR Difficulties Change in work processes. Attachment to paper. How well do you type? Staff acceptance VA (SNF) Orlando Using CPRS (Computerized Patient Record System). Fully integrated with VA Hospital, outpatient, lab, pharmacy, radiology, and other regional VA systems. Physician orientation – 2 formal half day sessions…plus on the job learning. To Overcome Barriers Multiple providers/ facilities Time – data entry Financial Maintenance of systems. Integration with other health care delivery systems. Hire a professional/product with SNF experience. Dedicate a project manager. Expect major change in work processes and culture shift. Time for training, money for incentives. 51 52 Successful Implementation Involves… For Staff Engagement Emphasize… Staff engagement and preparation. Working with partners and vendors. Adapting software to LTC environment. Managing the implementation. 53 Benefits to patient care. Eventual time savings. Reducing wasteful workflows. New skills. Need to do workflow analysis, identify champions, and start small with motivated people. 54 91 04 Medical Information Management For Partners and Vendors… Adapting to your Environment Take the time to review products with real life examples and exceptions. Go to places that have the system you are using and talk to users. Collaborate in teams to make vendors change. Means integrating software into clinician and physician workflow, both on and off site. Where and when do you enter and need information? How can you review it best? 55 56 Training Online Resources Available Has to be personalized, often one to one. Consider bringing the training to the physician’s office. Classroom training makes people leave thinking they know it, but they need hands on. Arrange ongoing support by someone who knows exceptions, even at night. 57 www.nursinghome.org/pro/HIT/hit.html HIS: Understanding the costs and benefits of health information technology in nursing homes and home health agencies 2009 includes many tool kits, articles from early adopters, basic facts about technologies and grants you can get. Medical Director Role in EMR E-Prescribing in LTC 58 No exception, but no certified systems. Increasing number of non-office/nonhospital based Physicians are using eprescribing, but Barrier – Facilities unlikely to allow physician to bypass nursing staff and communicate directly with dispensing pharmacy. Obtain reports for quality assurance. Advocate for MD use of EMR. 59 How will the doctors input? What data do doctors need to get from the record and is it easy? How does the record fit with the regular routine/work processes? (in person and off-site) Shows value as Medical Director. 60 92 04 Medical Information Management Change is coming, move with it. Best of Times – Stakeholders see great potential. HIPAA Worst of Times – So far away from a fully integrated EMR system. Health Insurance Portability and Accountability Act 61 62 HIPAA: What It Is HIPAA Goals Health Insurance Portability and Accountability Act Signed into law by President Clinton 08/21/96. Several Titles and Subtitles, etc. We are primarily concerned with parts of Title 2: the Privacy Rule and the Security Rule. HIPAA has many goals. The principle goal in which we are interested is Protect the privacy and security of patient information stored and exchanged electronically. 63 64 HIPAA: Privacy Privacy and Security Rules Privacy governs how patient-identifiable health information is used and disclosed Security governs how electronically stored or transmitted patient-identifiable health information is kept confidential by assuring the security and integrity of electronic health data. 65 The Privacy Rule addresses an individual’s rights to control access to and disclosure of his/her Individually Identifiable Healthcare Information (IIHI). IIHI is also more commonly referred to as Protected Health Information (PHI). PHI includes oral, written and electronic health data, past, present and future. 66 93 04 Medical Information Management Privacy Rule – IIHI is PHI: HIPAA: Use and Disclosure “Any information, whether written, stored in a computer or verbal . . . . Use that relates to the past, present or future health or condition of an individual, any healthcare services that individual has received, is receiving or will receive . . . TPO (Treatment, Payment, Operations) As permitted or required by the Rule Disclosure and which identifies the individual or there is reasonable basis to believe that the information can be used to identify the individual.” As permitted or required by the Rule As authorized by the person; standardized information required 67 68 ePHI HIPAA: Security Concerns only electronically stored or transmitted PHI, which is becoming more commonly referred to as ePHI. This is increasingly important because of the burgeoning use of EMRs and, with the current status of software, the risk of unauthorized disclosure of PHI. 69 Computer programs E-mail Billing information to insurers, Medicare, Medicaid Faxes Portable Communication Devices (phones, Blackberrys) Communication / Documentation tools such as Accunurse HITECH Act (2009) HITECH Health Information Technology for Economic and Clinical Health Act. Health Information Technology for Economic and Clinical Health Act 71 Part of the American Recovery and Reinvestment Act of 2009 (ARRA). Incentives related to health care information technology in general. Specific incentives aimed at increasing the use of electronic health record (EHR) systems among providers. 72 94 04 Medical Information Management HITECH Act – Important Items HITECH Act and HIPAA Widens the scope of privacy and security protections available under HIPAA. Increases the potential legal liability for non-compliance. Provides for more enforcement. Right to access to and copy of ePHI. Business Associates required to comply with the safeguards in the Security Rule. Imposes data breach notification requirements for unauthorized uses and disclosures of “unsecured [currently, unencrypted] PHI”. Increased enforcement provisions. 73 74 HIPAA and HITECH: Enforcement HIPAA and HITECH: Penalties Health Plans/Healthcare Clearinghouses /Healthcare Providers/Business Associates Enforcement is under the aegis of the Office of Civil Rights (OCR). New: 4 tiers of fault and penalties Violation category [42 USC 1320d-5(a)(1)] Minimum Penalty, each occurrence (A) Did not know and would not have known $100 - $50,000 (B) Reasonable cause and not willful neglect* $1,000- $50,000 (C) (i) Willful neglect* –corrected within 30 days of discovery $10,000 - $50,000 (C) (ii) Willful neglect* – not corrected $50,000 Willful neglect: “the conscious, intentional failure or reckless indifference to the obligation to comply" The maximum penalty for all violations of any category in a calendar year is $1.5M ($1,500,000). 75 76 HIPAA and HITECH: Penalties Health Plans/Healthcare Clearinghouses /Healthcare Providers/Business Associates New: 4 tiers of fault and penalties Violation category [42 USC 1320d5(a)(1)] (A) (B) HITECH: Criminal penalties Did not know and would not have known Reasonable cause and not willful neglect Minimum Penalty, each occurrence $100 - $50,000 $1,000- $50,000 (C) (i) Willful neglect – corrected within 30 days of discovery $10,000 - $50,000 (C) (ii) Willful neglect – not corrected $50,000 Before: if did not know and would not have known, penalty could be set aside; Now: Only defense against monetary penalties is for violations not due to willful neglect [Categories (A) and (B)] if the violations are corrected within 30 days of discovery. 77 Criminal penalties can now be enforced against individuals (including employees of a covered entity.) The scope of activities subject to criminal prosecution is broadened to include individuals who obtain or disclose individual PHI "without authorization." 78 95 04 Medical Information Management HIPAA Violation Slideshow Participants: Watch the following movie and make a list of HIPAA violations which are apparent in the movie. A series of photos are presented for 15 seconds each. Look at the photo and jot down the violations. 79 80 81 82 HIPAA Violation Movie Participants: Watch the movie and observe the clues to the HIPAA violations. Violations Discussion Critical Points Thinking about these scenarios, what are The Medical Director’s responsibility, and Corrective action the Medical Director might take to ensure that the violation(s) cease and do not recur. 83 Address HIPAA complaints promptly and aggressively. (Especially if you receive complaints from the Office of Civil Rights.) The potential civil penalties for violating HIPAA have been increased substantially via the HITECH act, and that these higher penalties can be applied to HIPAA violations of any kind. 84 96 04 Medical Information Management Critical points Medical Director Tasks Both covered entities and business associates: reevaluate all of HIPAA compliance practices, even for the parts of HIPAA that were not changed by HITECH. Provide or arrange for HIPAA education on an annual basis for medical staff. Maintain observatory vigilance about privacy protection. Report violations Insist on corrective action Always: advocate for our residents. More changes in enforcement will be forthcoming. 85 86 Small Group Exercises 4.6 – 4.8 87 Medical Information Management Review Wrap Up Breakout Example of system issue within a facility. Lack of compliance with policy and procedures. Physician oversight Quality assurance Solution multifaceted 88 Medical Record Components Systems Medical Director tasks The “survey friendly” medical record Regulatory compliant Facility staff Medical staff 89 90 97 04 Medical Information Management Medical Information Management Review Electronic data handling Current status Barriers to implementation Benefits HIPAA Role of the Medical Director Medical Information Management Review Tasks of the Medical Director Use of data to facilitate carrying out of Medical Director functions and tasks. 91 PHI (Protected Health Information) and IHII (Individually Identifiable Health Care Information) Engender a culture of protecting PHI. Ensure HIPAA education for the medical staff. 92 98 05 Employee Health and Safety Objectives 05 Employee Health and Safety Core Curriculum on Medical Direction Manage the potential ethical and legal conflicts resulting from establishing a physician-patient relationship with an employee while having a fiduciary relationship with the facility. List important occupational illnesses and injuries seen in the LTC setting. Describe components and processes of an effective employee health program. 2 1 Why Should You Know About Employee Health? Objectives Define the medical director’s responsibilities in developing a successful facility employee health program. Assess the adequacy of the employee health and safety program at the participant’s facility. Leadership Quality and Risk Management Federal and State Regulations Infection control 3 4 More Why? The NH is a Dangerous Place Why, Continued. You Have Much to Offer in the Employee Health Domain Medical Expertise Enhanced Moral Authority Medical Director 1.5 million NH employees Nursing homes among top 10 industries for musculoskeletal problems => Increased Employee Moral Nursing home workers (132 injuries per 1,000 workers Reduced Employee Turnover Worsening Workforce Issues Absenteeism & consequences of staff turnover, workers’ compensation claims, Comparable to airline baggage handlers, meat-packers Health-care frame of reference: 5 Hospital-based workers: 46 injuries per 1,000 workers. Home-care: 52 injuries per 100 workers but more severe Meyer et al. Am J Ind Med 1999; 35(3):295-301. Department of Labor, Bureau of Labor Statistics. May 2007 6 99 05 Employee Health and Safety A Case of Acute Shoulder Strain You are asked by the nurse to see Sarah, a CNA who has just “strained her shoulder” while lifting a resident. 5.1 7 8 A Case of an Acute Strain Liability Concern Can you see the employee in your facility? What if the employee is your office patient? What resources, practically speaking, are available to you in your setting? Medical Care of Employees is NOT an administrative duty, Therefore needs medical malpractice coverage. Document-Document-Document However, lawsuits related to treatment of an employee in the nursing home are extremely rare. 9 10 Another Liability Consideration Manage the Ethical Conflict … Female employees, keep pregnancy in mind as you order x-rays or prescribe. May not be at the forefront in a geriatric venue. You may want the facility to purchase a pregnancy test kit. The American College of Occupational and Environmental Medicine (ACOEM) Code of Ethical Conduct offers useful guidelines. MDs should: 11 Keep confidential all individual medical information. Release information only if required by: Law Overriding public health considerations. Other physicians according to accepted medical practice. At the request of the individual. 12 100 05 Employee Health and Safety Further ACOEM suggestions Manage the Ethical Conflict… The ACOEM Code states physicians should recognize that employers may be entitled to counsel about an individual’s medical work fitness, but not to diagnoses or specific details, except in compliance with laws and regulations. www.acoem.org/code 13 Workers should understand that their private disclosures will be treated in as confidential a manner as possible. Try to obtain consent before disclosing sensitive information, e.g. STDS such as hepatitis or HIV. Treatment for mental illness or substance abuse. If disclosure is legally required or consent is not legally required, the employee should be notified of the impending disclosure before the encounter. 14 A Workplace Clinic? Location Staffing Mid-level providers? Equipment Liability coverage Scope of practice What issues addressed What issues referred to PCP or for consultation Records Need written policy for the treatment of medical records: where and how the records are stored; Who has access? Mechanism of consent? What happens if employee leaves or facility closes? 15 Important Occupational Illnesses and Injuries Occupational Illnesses Primarily infectious diseases “Important” implies Common-ex. Low back pain The employee may contact at work or bring into the facility. Could cause serious morbidity or mortality for: Medically minor Impact by weight of numbers Uncommon-ex. Pulmonary TB, but potentially consequences for: Employee Facility and it’s residents Both Emerging-ex. SARS, H1N1 16 Employee Other employees Residents of facility 17 18 101 05 Employee Health and Safety Important Occupational Illness A Case of MRSA Blood Borne Pathogens: Hepatitis B and C, HIV GI: food and nonfood-related illness, Hepatitis A, Salmonella, Norovirus Respiratory: Influenza, TB Skin: Scabies, Zoster Two residents have been admitted with MRSA infections and active drainage. Staff has been reminded about the importance of hand washing, but now are hesitant to care for these two residents. The Director of Nursing asks you to assist in resolving the employee fears. How might you proceed? Note: covered in ID topic 19 20 Vaccination Case Your facility has a very low employee acceptance of influenza vaccination What would you do? 5.2 21 22 Flu Vaccination for Employees: Immunization Program Elements 5.3 23 A written facility policy and plan on immunization. An implementation manual. Training for staff members, including physicians, on the immunization plan. Evaluate by collecting and recording employee vaccination rates. 24 102 05 Employee Health and Safety Improving Vaccination Rates Barriers - Examples Engage leadership Standing orders Offer vaccine in workplace Free Consider incentives (door prizes, etc) Declination forms Evaluate progress Identify organizational & personal barriers Use QI process to test interventions Organizational barriers to better immunization performance: Inadequate vaccine supplies. General vaccine inaccessibility. Lack of positive incentives for immunization. Requirement of written consent. Limited record keeping. Lack of any feedback or shared learning. Individual barriers to better immunization performance: Limited leadership knowledge and support. Poor staff knowledge about influenza. Negative staff attitudes about the vaccine and injections. 25 26 Importance of Employee Vaccination Nace et al. JAMDA 2007; 8(2):128-33 Reduce transmission to vulnerable residents Reduce disruption to staffing Reduce mortality Improving employee vaccine rates to 50%60% => reduced flu mortality 40% Intervention used needs assessment to target address of these barriers => sustained 90% employee immunization rates Details: http://download.journals.elsevierhealth.co m/pdfs/journals/15258610/PIIS1525861006004919.pdf 27 28 Fig 1 QI Process Important Occupational Injury Source: JAMDA 2007; 8:128-133 (DOI:10.1016/j.jamda.2006.09.014 ) Copyright © 2007 American Medical Directors Association Terms and Conditions Musculoskeletal diseases “Stress” Latex Sensitivity Toxic exposures Workplace Violence 30 103 05 Employee Health and Safety Occupational Injuries Workman’s Compensation Assistive Equipment Employee Health and Safety 5.4 Ergonomic Guidelines 31 32 Back Injury - Epidemiology Back Injury Risk factors The most common musculoskeletal problem & cause of work-related disability in under 45 years of age. The most expensive cause of work-related disability due to workers' compensation and medical expenses. Most low back pain (97%) is mechanical and the majority of that is benign---especially in the age group of most employees. Deyo et al. NEMJ 2001; 344 (5):363-70 33 NH work is physically demanding which contributes to a high number of injuries. Injury is usually associated with manual lifting, transferring and repositioning of residents (particularly back injury). Awkward postures (e.g., working in confined areas) Large amounts of weight Unexpected shifting of weight Unexpected loading Employee factors Obesity Deconditioning Under-estimating the job 34 Prognosis 30-60% recover in 1 week, 60-90% recover in 6 weeks, 95% recover in 12 weeks. A Case of Back Injury However, relapses and recurrences are common. Good evidence that acetaminophen, nonsteroidal anti-inflammatory drugs, skeletal muscle relaxants, heat therapy, physical therapy, and advice to stay active are all effective. But…95% of back pain gets better regardless or in spite of interventions offered. AFP 2007; 8(7):1181-92 35 After the CNA Sarah was treated and quickly returned to work, the administrator mentions to you that low back pain is keeping employees off work for an average of 23 days and wonders if that period of disability can be safely reduced. What are some explanations? 36 104 05 Employee Health and Safety Reasons for Protracted Recovery Is the Problem Real? Disputed compensation claims. Fear avoidance (exaggerated pain or fear that activity will cause permanent damage.) Job dissatisfaction. Pending or past litigation related to the back pain. Psychological distress and depression. Reliance on passive treatments rather than active patient participation. Somatization AFP 2007; 8(7):1181-92 OR Is the data valid? Could this be a system problem? 38 Assistive Equipment and Prevention of Back Injury Not surprisingly, volume of back & musculoskeletal injuries increase WC costs. Assistive Equipment Does this figure sound reasonable? 37 Workman's Comp Can be ameliorated. Insurance and OSHA information have shown that Mechanical lifts work. Corsets and education are not effective. Recurrence reduced by Exercise programs of the back and legs that have aerobic conditioning and strengthening . Ergonomic redesign of strenuous job tasks. 39 40 Process for Addressing Ergonomic Issues OSHA Ergonomic Guidelines Provide management support. Involve employees. Identify problems. Implement solutions. Address reports of injuries. Provide training. Evaluate ergonomic efforts. Recommendations: Manual lifting of residents be minimized, and eliminated when feasible. Employers develop a process for systematically addressing ergonomic issues. http://www.osha.gov/ergonomics/guidelines/nursinghome/final_nh_guidelines.html 41 Weber. Nursing Management 2008; 39(7):28–31 42 105 05 Employee Health and Safety OSHA’s Occupational Hazards in LTC Nursing Home e-Tool http://www.osha.gov/SLTC/etools/nursin ghome/index.html Need Screen Shot - Thank You Click on the area for more specific information. Common safety and health topics: •Ergonomics •Patient Handling Program •Patient Handling Controls •Trips/Slips/Falls 43 •Awkward Postures •Other Ergonomic Hazards •Recordkeeping 44 In Sum-selected Injury Prevention Strategies from OSA Use mechanical lifts & other assistive devices. Raise bed Proper positioning of storage shelves & appliances-no reach or stoop. Esp. non-clinical staff, dietary, janitorial, laundry workers 5.5 – 5.7 Nursing stations: Ergonomically correct computer stations. Proper light to reduce glare, eyestrain. 45 46 Stress Stress on the Job On the job and brought from outside is important because: Compassion for employee. Distraction, inattentiveness can be related to errors and accidents. Probably a factor in staff to resident “abuse.” 47 Cognitively impaired residents. Family caregivers’ “advocacy.” Inadequate orientation, training, feedback. Violence/injury sustained at the hands of residents. 48 106 05 Employee Health and Safety Stress Brought From Outside Stress Reduction Psycho-social realities Family Responsibilities Domestic Violence Facility management possibilities: Job Supervisor Description & Training Job placement Action Plan Incentives Pay/Other positive reinforces NH social work role? Employee Assistance Plan (EAP) Financial issues Cultural issues Ethnic Diversity Language Barriers 49 50 51 52 Stress Reduction Societal responses: ADA (1991) Family Medical Leave Act (1993) ada.gov, www.dol.gov/esa Environmental Risks 53 Latex Cleaning and other Chemicals 54 107 05 Employee Health and Safety Latex Allergy: Allergic Contact Dermatitis Latex Allergy Irritant contact dermatitis (Nonimmune) Gradual onset, over days, caused by hand washing, occlusion, antiseptics and glove chemicals Symptoms - redness, cracks, fissures, scaling Type IV, delayed hypersensitivity Onset six to 48 hours after contact, caused by chemicals Symptoms - erythema, vesicles, papules, pruritus, blisters, crusting Am Fam Phys 2009 Am Fam Phys 2009; 80(12):1413-20. 55 56 Latex Allergy: Immediate hypersensitivity (type I) Case of the Chemical Spill Onset within minutes, very rarely longer than two hours, caused by latex Symptoms - urticaria, angioedema, nausea, vomiting, abdominal cramps, rhinoconjunctivitis, bronchospasm, anaphylactic shock Am Fam Phys 1998, 57 How will you respond? 58 MSDS Sheet Advise Bruce to remove clothing. Advise Bruce to wash with water. Ask the nurse to obtain for you the Material Safety Data Sheet (MSDS) for Envirocide. General first aid You are at the facility when a nurse runs you down. Bruce, a maintenance employee, just spilled “Envirocide” all over his pants. He is tearful & screaming for help. The nurse wants to know what to do. 57 How Did You Respond? Where are they kept in your facility? Be, or at least appear, calm and caring. 59 60 108 05 Employee Health and Safety MSDS Sheets MSDS Sheet - Envirocide 1. Identification 2. Composition information 3. Physical and chemical properties 4. Fire and explosion hazard data 5. Reactivity data 6. Health hazard data 7. Emergency first aid procedures 8. Precautions for safe handling & use 9. Control measures 10.Transportation information 11.Special information 6. Health hazard data Skin: Moderate irritation Eye: Contact with eyes can cause reversible damage Inhalation: Low or mild irritation 7. Emergency first aid procedures Skin: Wash skin with soap and water. 61 62 Employee Health Program: Components The Case of the Chemical Spill What about Prevention? Would this elicit an incident report in your facility? Would the report be reviewed in a thoughtful and meaningful manner? Who would do the review? Policies and Procedures Employee Health Nurse - “n of two” Committee Personnel Department Safety Committee Infection Control Committee Quality of Work Life (QWL) Committee 64 63 Employee Health & Safety Employee Health Program Components Employee Health Program: Tasks What role do you currently have in these areas? Hiring and Placement Monitoring & Surveillance of employee General Health Promotion Risk Management Prevention Do you have policies, personnel, or committees in place to address these issues? Are you, as medical director involved in any of these activities? Should you be? 65 66 109 05 Employee Health and Safety Employee Health Program: The Medical Director Role Employee Health Program: Tasks Educational Resource Hiring and Placement General Health Promotion Prevention Employee Health Program Risk Management Direct Care Provider Risk Manager Consultant To Administration Leader Monitoring & Surveillance Data Manager 67 68 Employee Health Resources, Summarized Medical Director Resources for an Employee Health Program Information Sources Communicable Disease Manual: Control of Communicable Diseases Manual Medical Texts and Journals Traditional texts and journals Dr. Pattee’s and Dr. Levenson’s textbooks JAMDA and Caring for the Ages Course Resource Disk An official report of the American Public Health Association 18th Edition 2006 Web pages www.acoem www.osha.gov www.cdc.gov www.ada.goc www.dol.gov 69 70 Medical Director Resources for an Employee Health Program A Parting “Meta-message” Scientific background Insider-outsider Knowledge Sources of Power Creditability Insurance Company Risk Management materials and consultants. Factual Expert 71 “Perhaps the most difficult shift for medical directors is making the shift from helping people directly and one-on-one to helping people indirectly through the creation and implementation of facility systems, policies and procedures, or educational ventures that help employees as a group.” Brechtelsbauer D. Caring for the Ages 2005; January 2005 72 110 05 Employee Health and Safety Employee Health & Safety Thank you for helping assure the health and safety of workers in America’s long term care facilities. 73 111 06 Infection Control Infection Control Module Objectives You will develop the knowledge and skills to: 06 Infection Control Core Curriculum on Medical Direction Develop or make recommendations for improving the infection control program in your facility. Help control and prevent important infectious illnesses dealt with in the LTC continuum, particularly nosocomial infections. State the regulatory basis for an infection control program. 2 Infection Control Module Objectives Infection Control Describe the medical director’s tasks that contribute to the facility’s infection control program. Access current regulations and clinical guidelines that impact this area of medical direction. Choose and utilize appropriate techniques and data sources to assist your facility in the monitoring of infectious illnesses. Why infection control? You are a part time medical director in a 100 bed facility. While busy in your office, with a full schedule of patients, you are handed a message from the facility ICP (infection control professional---in this case, as is true in most facilities, the ICP is a nurse who has many other duties in the facility). 3 4 Infection Control The note reads: “There was a needle stick incident at the nursing home. They need you to call the lab and let them know what labs are needed.” 6.1 5 6 112 06 Infection Control Where to Find Current, Authoritative, Regulatory Compliant Guidelines Textbooks or journal articles might be a good source, but most infectious disease recommendations ultimately come from the Center of Disease Control (CDC) and most regulatory issues, relative to employees, from the Occupational Safety and Health Administration (OSHA). Where to Find Current, Authoritative, Regulatory Compliant Guidelines Going to the appropriate web sites would produce precise, current, and authoritative recommendation regarding care of residents or employees potentially exposed, as in this case, to bloodborne pathogens. http://www.cdc.gov http://www.osha.gov http://www.shea-online.org http://www.apic.org//AM/Template.cfm?Section =Home1 7 8 Where to Find Current, Authoritative, Regulatory Compliant Guidelines A few clicks from either the CDC or OSHA website and you will find: Bloodborne Infectious Diseases: HIV/AIDS, Hepatitis B, Hepatitis C Emergency Needlestick Information http://www.cdc.gov/niosh/topics/bbp/emergnedl.html 9 Needle Stick at Nursing Facility What you find here is very likely to be current, credible, and helpful. As a short term solution can you refer the ICP to this resource and have him/her follow the stated guidelines? 12 113 06 Infection Control What do the Current Guidelines Say? What do the Current Guidelines Say? Factors to consider in assessing... Evaluation of occupational exposure sources Type of exposure Type and amount of fluid/tissue Infectious status of source Susceptibility of exposed person Susceptibility of the exposed person Hep B vaccine history, vaccine response status HBV, HCV, HIV immune status Test known sources for HBsAg, anti-HCV, and HIV antibody. Do not test discarded needles for bloodborne pathogens. For unknown sources, evaluate the likelihood of exposure to a source at high risk for infection. 13 14 Immunization of Health-Care Workers: Healthcare Professional Immunization Recommendations Strongly recommended by the CDC: 6.2-6.4 15 Hepatitis B Influenza MMR Varicella Tetnus, diptheria, pertussis Menigococcal www.immunize.org/catg.d/p2017.pdf (verified 4-2010) 16 Infection Control Next time you are in the facility, ask your ICP to bring you the Policy Manual regarding needle stick injuries and review it with him/her. 6.5 17 18 114 06 Infection Control 483.75 Administration 483.75 Administration F-Tag 501 (R) Medical Director The medical director is responsible for implementation of resident care policies. (IG) Admission, Discharge,Transfer Physician privileges and practices Non-physician health care workers Ancillary services Policies and procedures related to accidents and incidents [infection surveillance] F-Tag 501 (R) Medical Director responsible for coordination of resident’s care: (IG) Providing appropriate resident care. Monitoring and insuring implementation of resident care policies. Provide oversight and supervision of physician services and medical care. Oversee clinical care. Assuring support of essential consultants as needed. Regulatory Mandate: 483.65 Infection Control Infection Control Program F-Tag 441 (R) - Facility must: Must: Establish and maintain an Infection Control Program. Provide a safe, sanitary, comfortable environment. Help prevent development and transmission of disease and infection. Investigate, control, and prevent infections in the facility; Decide what procedures, such as isolation, should be applied to an individual resident; and Maintain a record of incidents and corrective actions related to infections. 21 Components of an Infection Control Program Program development and oversight Policies and procedures Documentation Infection control practitioner 483.65 Infection Control Infection Control Program (IG) Communicable disease reporting Education Antibiotic review Surveillance 22 Monitoring Data analysis 23 Defined in writing and include scope and application of infection surveillance, prevention and control program Program reviewed periodically Based on current standards of practice. 24 115 06 Infection Control Surveillance Infection Surveillance Program Facility should maintain a separate record on infection that identifies: 25 F-Tag 441 (IG) Infection Surveillance Program Must enable facility to timely analyze: Clusters Changes in prevalent organisms, or Increases in the rate of infection Pay attention to residents at high risk of infection: Pressure ulcers Nutrition compromised Invasive devices Immobile Recent GACH DC Incontinent 27 Surveillance data should be routinely reviewed and recommendations made for prevention and control of additional cases. F-Tag 441 (IG) 28 Infection Control Epidemiological Definitions Infection Control Surveillance Program 26 Infection Surveillance Program F-Tag 441 (IG) Each resident with an infection Date of infection (onset) Causative agent Site (of infection) Precautions taken to prevent spread It is important and useful to have precise definitions. Be sure you know what definitions the ICP is utilizing. Be sure the ICP is compulsive in adhering to definitions. 29 General rules: A. Only new symptoms or acute changes in chronic symptoms should be considered. B. Potential noninfectious causes of the symptoms and signs should always be considered before diagnosing infection. C. Infection should be diagnosed based on several supporting data and not on a single finding. Microbiological and radiological findings should be used only to confirm clinical evidence of infection. 30 116 06 Infection Control Infection Control Surveillance Program MDS 3.0 Infections present on the resident’s admission or readmission, or that develop within 72 hours after admission, are NOT considered Health Care associated infection (HAI). Aka nosocomial New guidance in 2009; previously 48 hours 31 Infection Control Diarrhea Outbreak Rates of infection: Calculation of rates. Review and trend monthly. Watch for patterns, outbreaks. Add your clinical knowledge to apparent statistical truth. Review of antibiotic usage is often an easily obtained and useful adjunct to ICP generated statistical data. Your facility has a significant diarrhea outbreak: Your Infection Control program is working (the medical director is supposed to be notified of possible outbreaks). The ICP calls to inform you that the facility has had 16 cases of diarrhea in the last two days. (No surprise, staff absenteeism is up too). Are there any recommendations to prevent spread you would like to make? 33 34 Preventing the Spread of Infection (R) The facility must require staff to wash their hands after each direct resident contact for which handwashing is indicated by accepted professional practice. (IG) Procedures must be followed to prevent cross contamination, including handwashing or changing gloves after providing personal care, or…. (IG) Facilities for hand washing must be available. 6.6 35 36 117 06 Infection Control Preventing the Spread of Infection Case 2: Diarrhea Outbreak When the infection control program determines that a resident needs isolation to prevent the spread of infection, the facility must isolate the resident. The facility must prohibit employees with a communicable disease or infected skin lesions from direct contact with residents or their food, if that direct contact will transmit the disease. You recommend isolation (remain in room, meals in room) for residents with diarrhea. This is of course in addition to your ongoing “standard precautions” policy. Your social worker says you can’t do that, it violates residents’ rights. 37 38 F-Tag 441 Preventing the Spread of Infection F-Tag 441 Preventing the Spread of Infection (R) When the infection control program determines that a resident needs isolation to prevent the spread of infection, the facility must isolate the resident. (IG) Isolate residents only to the degree needed to isolate the infecting organism. (IG) Method should be the least restrictive possible while maintaining the integrity of the process. (IG) Isolate appropriately to reduce the risk of transmission. 39 40 Diarrhea Outbreak Diarrhea Outbreak This example is “real.” In a South Dakota town between October 2, 2002 and January 8, 2003, 14% of 6093 residents became ill with acute gastrointestinal symptoms. In the facility, 56% of residents had gastrointestinal symptoms within a 9 day period at the end of December 2002. Investigation by the state health department strongly suggested that the majority of these cases were related to Norovirus infection. This is the same virus implicated in diarrhea outbreaks on cruise ships. 41 42 118 06 Infection Control Norovirus F-Tag 441 Handwashing High attack rates (68% in one study) Can shed up to two weeks after sx’s resolve Low infectious dose (< 100 virons) High persistence of agent in the environment Potential for multiple modes of transmission Percentage cases with vomiting > 50% Absence of long-lasting immunity Outbreaks can involve multiple strains www.cdc.gov/ncidod/dvrd/revb/gastro/norovirus-fact sheet-htm Your policy (and the SOM) says that the facility should follow the guidance for surveyors in F tag 441 for handwashing. 43 44 Infection Control There is a Guideline for Hand Hygiene in Health-Care Settings, dated October 25, 2002 from the CDC. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5116a1.htm Useful resource site: http://www.cdc.gov/handhygiene/Basics.html 6.7-6.12 45 46 Handwashing: Effects on Bacterial Load 5 4.5 4 3.5 Log reduction of 3 2.5 bacterial 2 counts 1.5 1 0.5 0 Clostridium Difficile 2010 – Clinical Practice Guidelines for Clostriduim Difficile Infection in Adults Soap and Water Alcohol-gels 15 30 60 Handwashing Time (seconds) 47 2010 Update by the Society for Healthcare Epidemiology in America and Infectious Disease society of America (Infection Control and Hospital Epidemiology May 2010, Vol 31(5) pp 431-455 ) http://azdhs.gov/phs/oids/epi/disease/cdif/documents/Clinical %20Practice%20Guidelines%20for%20C%20Diff%20Infection %20%202010%20update%20by%20SHEA-IDSA.pdf (accessed 4/30/11) 48 119 06 Infection Control Clostridium Difficile Clostridium Difficile Diagnostic Criteria Diagnostic Tests Diarrhea Evidence of CDAD by any of the following: Positive assay Pseudomembranous colitis Positive stool culture Cell Culture Cytotoxin Assay Combined with stool culture is “gold standard.” Long turnaround time. Stool Culture EIA PCR testing Sensitive but not specific. Rapid but less sensitive than cytotoxic assay. 49 C. difficile-associated Diarrhea Risk Factors in LTC Clostridium Difficile 50 Facts Leading cause of nosocomial enteric infection. 3 million new cases/year in U.S. 20 thousand new cases/year in U.S. outside hospital setting. 2003 nearly 2% of patients transferred from acute care to LTC had dx. 51 Low albumin Age Antibiotics Proton pump inhibitors More recent admission to the facility JAMDA 2005; 6:105-108 C. DIFF. 52 C. DIFF. 6.13 C. DIFF. 53 54 120 06 Infection Control Clostridium Difficile Clostridium Difficile Primary Prevention Primary Prevention Hand washing and gloves. What to wash with? Study – liquid soap vs 4% chlorhexidine Simple tasks but compliance low. Both proven to lower Clostridium difficile rates. Increasing pace of patient care (plight of the HCW). Responsibility (Epidemiology is your mother – “wash your hands”). Without gloves – no difference. With gloves – liquid soap out-performed 4% chlorhexidine. Wash with soap (Mom says: “Did you use soap?”). 55 56 Clostridium Difficile Room contamination rates (McFarland, 1989). C. diff. (-) patient = 8% C. diff. Asymptomatic carrier = 29% CDAD patient = 49% 6.14 57 58 Clostridium Difficile Primary Prevention Antibiotic Control 59 Avoid antibiotic use. Limit duration. 60 121 06 Infection Control Clostridium Difficile Clostridium Difficile Secondary Prevention Instruct visitors to hand wash with soap and water. Thorough cleaning of all contaminated and potentially contaminated surfaces. Private room with contact precautions. Hand washing/gloves. 1:10 bleach solution Maintain contact precautions for duration of diarrhea. Routine identification of asymptomatic carriers is not recommended. 61 If single room not available cohort providing commode for each resident. Treatment of these asymptomatic residents is not recommended. 62 Infection Control Influenza The ICP calls you at the office. Three residents in your facility have malaise, headache, cough, and fever of 101.2 or higher. Influenza, at least according to the local TV station, is prevalent in the community. Your facility succeeded in administering influenza vaccine 8 weeks earlier to 97% of residents and 68% of employees and volunteers. 63 64 Things to Consider about Instituting Chemoprophylaxis: 6.15 – 6.18 65 Amantadine and rimantadine no longer recommended due to high resistance rates . Neuraminidase inhibitors oseltamivir or zenamivir? Are some folks already on treatment from their attending physicians? If there’s an outbreak---are drugs in the quantity you need available? Do some folks need liquid formulations? 66 122 06 Infection Control Things to Consider about Instituting Chemoprophylaxis: Things to Consider about Instituting Chemoprophylaxis: Do you need to know, or does the pharmacy insist on being informed of the serum creatinine (ozseltamivir or adamantanes)? Do you have a problem list that will identify COPD or Asthma (zenamivir)? Do you need to notify the POA for any or all residents? Will you do any active monitoring for ADRs related to the prophylactic medication? How much will all this cost? Who will pay? (Patient? Part D carrier? Facility?) How long do you need to continue prophylaxis once started? Are there any drug / disease interactions that would preclude use of the first choice drug? 67 68 483.25(n) Influenza and Pneumococcal Vaccinations www.cdc.gov/flu/ (accessed 4/30/11) 69 70 Vaccinations Influenza Vaccine October 7, 2005 Federal Register / Vol. 70, No. 194 / Friday, October 7, 2005 / Rules and Regulations. Pp 58834-58852 Pneumococcal Polylsaccharide Vaccine (PPV23) Influenza Vaccine Recommended annually for, among others, those living and working in LTC facilities. As of October 2002 CMS permits a standing order program for PPV and influenza vaccines. Revaccinate annually. Detailed recommendations published annually by CDC. Quality Measure (Short and Long Stay residents) 71 Vaccinations Pneumococcal Polysaccharide Vaccine (PPV) Recommended for, among others, those living in LTC facilities. As of October 2002 CMS permits a standing order program for PPV and influenza vaccines. Revaccinate once, five years after the first dose, if first dose was given before age 65. Quality Measure (Short and Long Stay residents) 72 123 06 Infection Control Vaccinations Herpes Zoster Vaccine Approved (FDA) for individuals over age 50 (ACIP recommendation not yet extended below age 60) Not reimbursed by Medicare Infection Control – Part II Some part D carriers will cover Not used for the treatment of Zoster Not used for the treatment of post herpetic neuralgia Tetanus 75 76 Burden of Infections Among U.S. Nursing Home Residents Resistant Organisms * wound infections, respiratory infections, urinary tract infections, or pneumonia Centers for Medicare and Medicaid Services, Long Term Care Minimum Data Set, Resident Profile Table as of 05/02/2005. Baltimore, MD 77 78 124 06 Infection Control Prevalence of Resistant Organisms Upon Admit to Hospital from NFs By site Resistant Organisms Urine Blood Wound Sputum Gram positive Gram negative MRSA (24%) ESBL-producing K. Pneumoniae (18%) ESBL-producing E. coli (15%) VRE (3.5%). Only 6% of patients with resistant organisms were on infection control precautions at the time of the survey cultures High ADL dependence was a predictor of MRSA and ESBL-producing Klebsiella. Prior Abx was predictive of MRSA and VRE. By organism class Trick et al have reported from a point-prevalence survey (using rectal, nasal, GI-tube site, wound, and axillary cultures) in a skilled nursing facility that 43% or residents had one or more of: 17% 7% 52% 40% 19% (almost all MRSA) 3% Am J Infect Control 2001; 29(3):139-44 79 80 JAGS 2001 Mar; 49(3):270-6. Resistant Organisms The admissions coordinator wants to admit a patient whose labs indicate MRSA is growing in the sputum. The ICP calls to see if this is ok and to ask what precautions, if any, will be necessary. 6.19-6.23 81 82 Staphylococcus aureus Colonization in Nursing Home Residents Staphylococcus aureus Colonization in Nursing Home Residents 213 residents of MI NF’s in a prevalence study (nares, oropharynx, groin, perianal, wound, and enteral feeding tube site cultures). 62% were colonized with MRSA. 75% colonized among those with indwelling devices. 49% colonized among those without indwelling devices. Nares cx’s were positive in only 65% of those with MRSA colonization. Clin Infect Dis 2008 May 1; 46(9):1368-73 83 Colonization is often transient. Persistence may be associated with density on semi-quantitative cx’s. (Infect Control Hosp Epidemiol 2008; 29(2):143-8) MRSA less likely to be persistent than MSSA. (Am J Infect Control 1997;25(4):312-21) 84 125 06 Infection Control Preventing Transmission of MRSA in LTC 2008 Cochrane review: 6.24-6.25 85 There is no evidence in LTC that screening potential admissions for MRSA or decolonizing those who are positive reduce LTC colonization or infection rates. Yashikowa and Strausbaugh, 2007 MRSA in LTC Looking for nosocomial spread Drinka looked at Staph aureus isolates from a VA Nursing Home in WI. A shift in abx use from TMP-sulfa to quinolones was associated with an increase in MRSA isolates. (22%51% between 1997 and 2002). MSSA and MRSA usually sensitive to TMPSx. MSSA usually sensitive to quinolones. MRSA usually resistant to quinolones. 87 88 MRSA in Wounds 86 Antimicrobial Use and MRSA Lee et al demonstrated in a NF that antibiograms alone or genotyping alone were each associated with a poor discriminatory ability. Many organisms with same genotype had variable antibiotic sensitivity patterns. Many organisms with same antibiogram had different genotypes. The two techniques are likely to be complimentary in investigation of potential epidemic spread. Infect Control Hosp Epidemiol 2000;21(3):218-21 No studies met the review criteria. Much of the evidence for recently-issued United Kingdom guidelines for control and prevention of MRSA in health care facilities was generated in acute care settings. It may not be possible to transfer such strategies directly to the nursing home environment. Cochrane Database Syst Rev 2008 Jan 23;(1):CD006:354 MRSA in Urine Attempt to cohort with other MRSA residents Avoid non-MRSA roommates who have unhealed wounds, indwelling catheters, or are immunosuppressed If drainage can be contained in a dressing, resident may go out of room unless exhibiting behaviors likely to increase chance of transmission (e.g., picking at wound dressing, picking nose) 89 MRSA in urine Cohort with other MRSA patients. Avoid high risk roommates. If continent, may leave room. If incontinent, ICP and Medical Director should analyze whether isolation to room is necessary (usually not). 90 126 06 Infection Control Respiratory MRSA Active pneumonia or bronchitis MRSA Private room Standard surgical masks for all entering room. Respiratory tract colonization without signs of infection. Private room not necessary. Cohort Avoid high risk roommates. At first sign of acute exacerbation, re-evaluate need for respiratory (droplet) isolation. 91 Housekeeping: standard practices appropriate Barriers: Gloves should be used, wash hands after removing gloves. Gown use if care activity likely to result in soiled clothing. (i.e., gown not needed to take a temperature or give medication.) Masks needed only if aerosolization likely. Isolation carts likely to be helpful. 92 Evolution of Drug Resistance in S. aureus MRSA in LTC In LTC S. aureus Infection rates colonized=10%/yr Non colonized =2-4%/yr Colonization not clearly related to MRSAinduced morbidity. Non-MRSA mortality in colonized residents is 2-3 times higher than in non-colonized (probably reflecting functional status and underlying disease). 93 [1997] Vancomycin [1990s] Vancomycin- resistant S. aureus [ 2002 ] Vancomycin intermediateresistant S. aureus (VISA) Vancomycin-resistant enterococci (VRE) 94 What We Think We Know About VRE Resistant Organisms Penicillin Methicillin MethicillinPenicillin-resistant resistant [1950s] [1970s] S. aureus S. aureus (MRSA) What if the potential resident had a urine culture showing VRE? Would you approve admission? Enterococci, (E. faecalis & E. faecium) 95 Normal inhabitants of the bowel. Often resistant to aminoglycosides. When high resistance occurs to gentamycin and streptomycin, there is usually no reliably bactericidal regimen. 96 127 06 Infection Control What We Think We Know About VRE Multiple genetic mechanisms for vancomycin resistance. Vancomycin resistance has been demonstrated to transfer between VRE and Staph aureus, Listeria, and Strep pyogenes. Death rates from VRE bacteremia may exceed 30%. What We Think We Know About VRE Risk factors for colonization Recent treatment with oral or parenteral Vancomycin or cephalosporins. Recent treatment with anti-anaerobic drugs (metronidazole, clindamycin, imipenem). Prolonged hospitalization. Proximity to patient colonized by VRE (not clearly demonstrated in LTC). 97 98 What We Think We Know About VRE Risk factors for colonization What We Think We Know About VRE Care by nurse who cares for another VRE patient (documented in Acute Care). Longer ICU stay. Care in hospital with high VRE prevalence. Contamination from inanimate objects. Factors increasing environmental or skin contamination (e.g., diarrhea). Colonization Fecal VRE an important source of infection as well as nosocomial spread. Skin colonization (even above the waist) is common. Duration of colonization variable (up to years). 99 What We Think We Know About VRE NF residents colonized with VRE are at increased risk of colonization/infection with other resistant organisms. Rectal colonization with MDR Gm neg 17 % vs 4% 6 month rate of subsequent CDAD 26% vs 2% 6 month rate of subsequent MRSA infection 17% vs 4% Infect Control Hosp Epidemiol 2003; 24(4):242-45 101 100 Control Efforts for VRE Limit use of vancomycin. Limit use of other antibiotics, especially cephalosporins. Vigorous environmental cleaning. Isolation Rarely eliminate VRE entirely from institution. 102 128 06 Infection Control What We Think We Know About VRE Inappropriate Uses of Vancomycin Control Efforts Consider Medical Director chart review of residents with orders for vancomycin, fosfomycin, quinupristin-dalfopristin and linezolid to ensure drug is truly indicated. Eradication of MRSA colonization. Primary treatment of C. difficile colitis. Prophylaxis for indwelling catheters. Topical use for irrigation. When cultures are negative for B-lactam resistant organisms. When only 1 of multiple blood cx’s “+” for coagulase negative staphylococci. 103 104 Appropriate Uses of Vancomycin Treatment of serious infections caused by beta-lactam resistant gram positive organisms. Treatment of infections caused by gram positive organisms in patients with true beta-lactam allergy. C. difficile colitis which is both severe and unresponsive to metronidazole. VRE Control Efforts All enterococcus isolates should be tested for sensitivity to vancomycin (check to determine that your lab does). Surveillance cultures for VRE are NOT indicated unless in epidemic situation, or high risk unit (vent unit, dialysis unit). Do stool or rectal swab culture on roommates of newly diagnosed VRE residents. http://www.cdc.gov/ncidod/dhqp/ar_multidrugFAQ.html#5 105 106 VRE Control Efforts Dedicated equipment VRE Control Efforts: Isolation Blood pressure cuff, thermometer, steth Notify ambulance staff and receiving hospitals/clinics when VRE resident is being transferred. Educate staff about VRE and facility’s VRE policies. Monitor rates of VRE infection and compliance with policies. 107 Private room or cohort with another VRE patient . Wear gloves when entering room of VRE resident. Wear gown if substantial contact with resident or environmental surfaces is anticipated, if resident is incontinent, or resident has ostomy, diarrhea, or wound drainage. 108 129 06 Infection Control Preventing Transmission of Resistant Organisms in LTCFs Trick et al compared 2 strategies: Preventing Transmission of Resistant Organisms in LTCFs Routine glove use without contact isolation Contact isolation No difference in baseline prevalence of resistant organisms [MRSA 21%; ESBL Klebsiella 14-17%; VRE 14-19% with prevalence slightly higher in glove use unit]; ESBL E coli was more prevalent on glove use unit (25 vs 12%). J Am Geriatr Soc 2004; 52:2003-2009 109 J Am Geriatr Soc 2004; 52:2003-2009 VRE Control Efforts: Isolation Devoted equipment in room. Remove gloves immediately upon exiting room AND wash hands with soap and water. Ensure clothing and hands don’t contact environmental surfaces after removal of gloves and gown and handwashing. Stopping VRE Isolation No proven recommendations for LTC. Consider: Primary site culture is negative x1 if site is normally sterile. Primary site culture is negative x2 (at least 72 hours apart) if site not normally sterile (e.g., skin, bowel,). Stool VRE cultures negative x3 (at least 72 hours apart). 112 VRE Control Efforts: Isolation Emerging Resistance SHEA Isolation Recommendations Limit resident transport to situations required for medical care; transport with precautions. Residents may travel out of room, assuming they are coherent (able to understand instructions about basic hygiene), continent of stool (or diapered to contain stool), and wearing clean clothing. Room restrictions probably appropriate for residents with wound drainage not contained by a dressing, or those incontinent or having diarrhea. Infection Control and Hospital Epidemiol Vol. 19, No. 7, Jul., 1998 110 VRE Control Efforts: Isolation 111 No significant difference in rates of acquisition of resistant organisms occurred: Rate/1000 patient days = 1.5 for Routine Glove Use 1.6 for Contact Precautions Hand hygiene occurred more frequently in the Glove group 57% vs 36% of observations (p = 0.02). Costs of Contact Precautions were 40% higher. 113 Multi drug resistance Acinetobacter ESBL gram negatives Carbipenem-resistant Enterobacteriaceae 114 130 06 Infection Control Emerging Resistance Emerging Resistance Multi-Resistant Gram Negatives Resistant to all of: Ceftriaxone Cefapime Piperacillin Gentamycin Tobramycin OR They have documented ESBL (extended broad spectrum beta-lactamase production) [note: this is only possible at this time with E. coli and Klebsiella. More likely to emerge on anti-anaerobic Abx regimens (Infect Control Hosp Epidemiol 2003;24(9):644-9) Multi drug resistant Acinetobacter baumannii Resistant to all antimicrobial agents or all except imipenem. Risk factors (Am J Infect Control 2002;30(7):386-90) Prior Abx Prior hospitalization Prior NF residency Use of a vent or trach Foley 115 116 Resistant Organisms in NonHospital Settings: CDC Guidance XDR Acinetobacter Acenitobacter baumanii Gram negative Most commonly encountered as a health-care acquired infection. As with most other organisms, treatment should only be given for infections (Not for colonization). Isolation: Enhanced contact precautions (appropriate hand washing, gloving, and gowning). 117 Standard and Contact precautions; and consider: Patient placement - Private room, if possible. (when not available, cohort). Another option is to place an infected patient with a patient who does not have risk factors for infection. Group activities – Maintaining socialization and access to rehab is important. Infected or colonized patients should be permitted to participate in group meals and activities if draining wounds are covered, bodily fluids are contained, and the patients observe good hygienic practices. http://www.cdc.gov/ncidod/dhqp/ar_multidrugFAQ.html#5 Accessed 5/1/11 118 Tuberculosis in LTC Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005 (MMWR) Facility risk assessment determines facility program. Criteria for the Frequency of TB screening for HCWs and residents has been changed. 119 131 06 Infection Control TB Control Measures Administrative Environmental Respiratory protection 2005 Updated Guidelines on TB Prevention in Health Care Settings MMWR December 30, 2005 / Vol. 54 / No. RR-17 TB Control Administrative Measures Assign responsibility for TB infection control in the nursing home. Conduct TB risk assessment. Develop / institute written TB infection-control plan to ensure prompt detection. airborne precautions. treatment of persons who have suspected or confirmed TB disease. Ensure timely availability of recommended laboratory processing, testing, and reporting of results to the ordering physician. Implement effective work practices for the management of patients with suspected or confirmed TB disease. 125 124 TB Control Administrative Measures Ensure proper cleaning and sterilization or disinfection of potentially contaminated equipment (e.g., bronchoscopes, endoscopes); (not usually an issue for LTC). Train and educate health-care workers (HCWs) regarding TB, with specific focus on prevention, transmission, and symptoms. Screen / evaluate HCWs who are at risk for TB disease or who might be exposed to M. tuberculosis (TST). Apply epidemiologic-based prevention principles, including the use of setting-related infection-control data. Use appropriate signage advising respiratory hygiene and cough etiquette. Coordinate efforts with the local or state health department. 126 132 06 Infection Control TB Control Environmental Measures Facility Risk Assessment Use of environmental controls to prevent the spread and reduce the concentration of infectious droplet nuclei in ambient air. Primary environmental controls control the source of infection by using local exhaust ventilation (hoods, tents, or booths) and dilute and remove contaminated air by using general ventilation. Secondary environmental controls control the airflow to prevent contamination of air in areas adjacent to the source (airborne infection isolation [AII] rooms) and clean the air by using high efficiency particulate air (HEPA) filtration, or ultraviolet germicidal irradiation. 127 Considers Classifies facilities Assesses screening (TST / BAMT) and conversion rates. Assesses the presence and performance of the infection control program and the presence of a person identified as responsible for the program. Looks at Environmental Controls and Respiratory Protection Program. http://www.cdc.gov/tb/publications/guidelines/AppendixB_092706.pdf accessed 5/1/11 Baseline two-step TST testing required. Periodic repeat TST testing interval now varies, based on the facility’s risk assessment (though states may require greater frequency than the feds). HCWs refer to all paid and unpaid persons working in healthcare settings who have the potential for exposure to M. tuberculosis. HCWs who have duties that involve face-to-face contact with patients with suspected or confirmed TB disease (including transport staff) should be included in a TB screening program. 129 130 Reading the Tuberculin Skin Test 128 TB Screening Program Health Care Workers Employee Health - TST Baseline Testing and Periodic Retesting Low risk Intermediate risk Ongoing Transmission Infection Control TB incidence in facility, community, and state. Whether facility treats patients with TB. Read reaction 48-72 hours after injection. Measure only induration. Measure the greatest length of induration perpendicular to the long axis of induration. Record reaction (induration; not redness) in millimeters. Infection Control TB You are asked to look at what the ICP feels is an equivocal TB skin test 131 Having instructed and observed the ICP administer PPD testing, you are confident there were no technique problems. You feel the test has 11 mm induration (and 17 mm erythema, but that doesn’t count). 132 133 06 Infection Control Negative TST Anergy If there is <5 mm of induration or no reaction at all, the test is considered negative. Do not rule out diagnosis based on negative skin test result. Consider anergy in persons with no reaction if: Always record the test results in millimeters (mm) and not as “negative”. 133 Boosting HIV infected Overwhelming TB disease Severe or febrile illness Viral infections Live-virus vaccinations Immunosuppressive therapy Anergy skin testing no longer routinely recommended. Two-Step Testing Some people with LTBI may have negative skin test reaction when tested years after infection. Initial skin test may stimulate (boost) ability to react to tuberculin. Positive reactions to subsequent tests may be misinterpreted as a new infection. Use two-step testing for initial skin testing of adults who will be retested periodically If first test positive, consider the person infected. If first test negative, give second test 1-3 weeks later. If second test positive, consider person infected. If second test negative, consider person uninfected. 135 136 Positive TST > 5mm 134 Positive TST > 10mm HIV infection Close contact with an infectious tuberculosis case in past year. Chest x-rays with fibrotic lesions likely to represent healed TB. Organ transplant / immunosuppressed (> 15 mg Prednisone/day for > 1 month). Receiving Tx with TNF-alpha antogonists. 137 >10 mm is classified as a positive reaction in all other persons who do not meet the above criteria but who have other risk factors for TB, including: Recent immigrants (i.e., w/in the past 5 years) from countries with a high prevalence of TB such as Africa, Asia, Eastern Europe, Russia, and Latin America. Injection Drug Users Residents / employees of high-risk congregate settings: Nursing homes and other LTC facilities for the elderly. Hospitals and other health-care facilities, and homeless shelters. 138 134 06 Infection Control Infection Control Infection Control TB You confirm that there is 11 mm of induration; therefore you would likely recommend further evaluation and treatment for this new employee. You are asked to look at what the ICP feels is an equivocal TB skin test. The test was on an newly employed CNA, a refugee from Bosnia. She had BCG when she was in school about 11 years ago. Is BGG a contraindication for TST? She has had no weight loss, cough, chills or sweats, and had not knowingly been exposed to anyone with TB. If evaluation is reassuring (i.e., no active TB), the recommended treatment is 9 months of INH (for INH-resistant, see appendix). 139 140 BCG Vaccination and Tuberculin Skin Testing Infection Control Tuberculin skin testing not contraindicated for BCGvaccinated persons. LTBI diagnosis and treatment for LTBI considered for any BCG-vaccinated person whose skin test reaction is > 10 mm, if any of these circumstances are present: Was contact of another person with infectious TB. Was born or has resided in a high TB prevalence country. Is continually exposed to populations where TB prevalence is high. Your DON approaches you frantically indicating that she needs to fill a nursing vacancy, and has a nurse from the Philippines who has a green card and comes with good references, but the nurse states she previously had BCG refuses to have a TST despite sharing the info discussed above. Are there any other options? 141 142 2005 Updated Guidelines on TB Prevention TB Screening Program Blood Assays for M. tuberculosis The whole-blood interferon gamma release assay (IGRA), QuantiFERON®-TB Gold test (QFT-G) FDA approved in vitro cytokine-based assay for cell-mediated immune reactivity to M. tuberculosis. Might be used instead of TST in TB screening programs for health care workers. An example of a blood assay for M. tuberculosis (BAMT). MMWR December 30, 2005 / Vol. 54 / No. RR-17 143 The QFT-G measures cell-mediated immune responses to peptides from two M. tuberculosis proteins that are not present in any Bacille Calmette-Guérin (BCG) vaccine strain and that are absent from the majority of nontuberculous mycobacteria (NTM), also known as mycobacteria other than TB (MOTT). http://www.cdc.gov/nchstp/tb/pubs/mmwrhtml/Maj _guide/Diagnosis.htm 144 135 06 Infection Control Summary Facility must maintain an active infection control program. 6.26 Surveillance-systematic data collection to identify infections in residents. Outbreak control-system for detection, investigation, and control of epidemics. Isolation-an isolation and precautions system to reduce the risk of transmission of infectious agents. 145 146 Establishing / Updating Infection Control Program Summary Set Deadlines Policies and procedures-relevant to infection control. Education-continuing education in infection prevention and control. Antibiotic stewardship-a system for antibiotic review and control. Employee health program Performance improvement/resident safety (QA&A) Deadline #1: Complete / Review and Revise manual Deadline #2: Establish permanent Committee and IC Coordinator Deadline #3: Train Committee and Coordinator Deadline #4: Inservice Everyone Deadline #5: Implement program 147 Establishing / Updating Infection Control Program: No Surprises Communicate with stakeholders Infection Control Administration ($) Director of Nursing Infection Control Coordinator Medical Staff Board of Trustees Werner’s rule: “If someone would be surprised to hear from anyone but you, talk to that one first.” Infection Control - Parting Thoughts Get to know the facility ICP very well. Be sure this person is well-trained and trustworthy. Policies may seem boring, but they can really help people keep their wits and do the correct thing in a time of urgency or crisis * 150 136 06 Infection Control Infection Control Infection Control Infection Control - Parting Thoughts Guidelines may seem set in stone, but they change frequently. Develop a method to keep up with changes. Distinguish between proposed changes (which the administrator hears about and panics) and actual finalized changes (which, if enforced by regulation, must be accommodated). Infection Control - Parting Thoughts Recognize not all guidelines are written with the realities of LTC in mind. Sometimes the guidelines are enforced by regulation and you must make them work. Sometimes logical thinking is permissible and adaptation is appropriate and acceptable. 151 Infection Control 152 Breakout Session Infection Control - Useful References www.cdc.gov…consider subscribing to MMWR www.osha.gov Control of Communicable Diseases Manual, an official report of the American Public Health Association, 19th edition, 2008 David L Heymann, editor 153 154 137 07 Resident Rights Learning Objectives 07 Resident Rights Core Curriculum on Medical Direction 1 Enumerate basic categories of resident’s rights. Discuss factors that influence the ability of residents to exercise their rights. Describe common situations where Resident Rights are relevant. Discuss the prevention of and response to abuse and neglect. Compare and contrast the Medical Director’s role and the Attending Physician’s role in honoring Resident Rights. 2 Resident Rights RIGHT F Tag Fed Regulation F150, 241, 242 483.10 483.15a and b Telephone & Mail F183,184,187 483.10 (i) (k) Protection of Funds F159,160,172 483.10(c) Complaints/Grievances F177,178 483.10(f)(l) Physician Services F385-390 483.10(d)(1) 483.25(l)(1) 483.40 Medication Usage F155,329,428,429 483.10(b)(4) 483.25(l)(2) 483.60(c)(1)(2) Basic Resident Rights Case 1: Rules for Admission and Notification 3 4 Admissions May not be Denied Based on…. Race Constitutional Rights Political Beliefs Sexual Orientation Religion* Admission Marital Status Age National Origin Admission May Not be Based Upon . . . Payment guarantee by another. Gifts or donations. Medicaid or Medicare benefits. Disability 5 Ineligibility Refusing to apply. Waiving benefit rights. 6 138 07 Resident Rights Facility Must Notify the Resident of These Rights: Reasons for Admission Denial No bed available. Cannot meet resident’s needs. No Medicaid bed available or facility is not Medicaid certified. Private religious/fraternal organization. Medical treatment Resident has the right to refuse any or all treatment. Advance directive Resident has right to choose or refuse to have an advance directive. Resident has right to choose personal representative. 7 Notification Responsibilities, Cont. Notification Responsibilities, Cont. 8 Written notice of services and their charges. Copy of all his/her bills. Any room or roommate change, or decision to transfer or discharge. Recent survey within last 5 years and the last plan of correction. Physician information The PASRR Termination of Medicare benefit Medicaid Rights: Determination of eligibility. Receive information on how to apply for coverage. Right to appeal decisions. 9 Notification Responsibilities, Process Immediate Notification How? Orally and in writing and signed by resident/responsible party. In a language the resident understands (e.g. sign language). When? 10 Accident results in injury. Significant change in physical, mental, or social status occurs. There is a need to significantly alter treatment/ care plan. Upon admission, change of regulations or resident request. 11 12 139 07 Resident Rights Case 2 Re-admission, Transfer and Discharge Rights Readmission Resident still requires nursing care. Appropriate bed is available. If hospital stay is >3 over nights and resident qualifies for Medicare, resident must use Medicare benefit. 13 Involuntary Discharge or Transfer Transfer and Discharge Medical Reason NF Loses Medicaid Danger Involuntary Move NF Closes 14 Bills not paid Advance notice of room or roommate changes. Refuse transfer if for Medicare reimbursement. Discharge self at any time unless a guardian has been appointed. NF No Longer Required 15 16 17 18 Complaints May complain/appeal decision of transfer or discharge. Without fear of retaliation, harassment, or eviction. NF’s complaint procedure. May complain to anyone. 140 07 Resident Rights Complaints The Care Plan All names, addresses, and phone numbers of pertinent state client advocacy groups should be posted: Resident participation State survey and certification agency State licensure office State ombudsman program Medicaid fraud and control unit Language the resident understands. Right to access the care plan and other records. Notification Information in advance about proposed care or changes to care. Immediate notification of change. 19 The Care Plan Privacy Rights Servicing residents so that they reach and maintain the highest practicable. 20 Physical Mental Social well-being Preventing decline when possible. A private room only if medically necessary Medical treatment Written communications Phone conversations Visitation Mail 21 Civil and Constitutional Rights 22 Representation Right to vote. Right to make informed decisions before consent. Freedom from discrimination. Right to meet privately to discuss issues. 23 Right to Identify Representative to Act on One’s Behalf 24 141 07 Resident Rights Case 3 Person Centered Care Right to Work Regulations Right to refuse to work. Must be permitted by Care Plan. Paid or voluntary. 26 25 Person-Directed Care Person-Directed Care 9th function 6 associated tasks CQI to ensure quality of care in persondirected care initiatives. Encourage active participation of residents in their plan of care. Development of policies and procedures that ensure that residents are provided with choice. Collaborates with IDT, family and allied services to ensure person-directed care. Educates medical professionals on individualized care. Collaborates with nursing home leadership to create an empowered person-directed care environment. 27 28 Quality of Life Resident Rights include… Person-Directed Care F 241 Dignity “No signs posted that include confidential or personal information.” Self-determination F 242 Self-Determination and Participation Dignity Participation in activities Accommodation of needs “Residents must have choices over their daily routine including –” Waking/Sleeping Bathing Eating Association 29 30 142 07 Resident Rights Quality of Life Resident Rights include … Quality of Medical Care Visiting hours Personal possessions Clean and “homelike living space.” Access to stamps and writing materials. Physician Services (F385-F390) Right to choose physician. Right to know credentials of physician. Right to change physicians. Right to expect standard of care. 31 32 Quality of Medical Care: Medications Quality of Medical Care Physician Services (F385-F390) To be seen q 30 days for 1st 3 months, then q 60 days, and as medical needs dictate. Physician responsible for initial comprehensive visit. Right to self-administer medications. May not apply to residents of private nursing facilities. 33 34 Quality of Medical Care: Unnecessary Drugs (F329) Right to Refuse Treatment Excess doses. Excessive periods of time. Without adequate monitoring. Producing adverse effects that indicate the drug needs. Reduced Stopped 35 Resident has right to refuse, by informed consent, if there is decision-making capacity. If refusal prevents proper care according to professional standards, discharge may occur after appropriate notice. Right to refuse to take part in any clinical research procedures, without jeopardizing resident’s care or stay in the facility. 36 143 07 Resident Rights Case 4: Bill & Effie Rights of Elders Constitutional guarantees: Constitutional rights are not changed because of a change of living situation. Resident Rights vs. Facility Responsibilities Involving Sexual Behavior Three components required for consent: Comprehension Consequences Choice Communicate Consistent Facility/community responsibility: To protect vulnerable elders from harm. To prevent abuse or assault. To monitor elders in declining health, especially those with cognitive impairment. Suggested Clinical Queries Patient’s awareness of the relationship: Is the patient aware of who is initiating sexual contact? Does the patient believe that the other person is a spouse and, thus, acquiesces out of a delusional belief,? Can the patient state what level of sexual intimacy [he/she] would be comfortable with? Limits: Diminished mental capacity and cannot consent. Most Widely Accepted Criteria for Consent Conflict Freedom of expression Freedom of association Pursuit of happiness Suggested Clinical Queries Patient’s ability to avoid exploitation: Is the behavior consistent with formerly held beliefs/values? Does the patient have the capacity to say no? Patient’s awareness of potential risks: Does the patient realize that relationship may be time limited? Can the patient describe how [he/she] will react when the relationship ends?” Lichtenberg, Strzepek. The Gerontologist 1990; 30:117-20. 144 07 Resident Rights Case 5 Abuse & Neglect Abuse and Neglect Neglect Mental, physical, sexual, and verbal abuse Punishment Misuse of property 43 Barriers to Reporting Abuse: Attending Physician Abuse and Neglect: Medical Director’s Responsibilities Concerns about alienating. 44 Family Facility personnel Easy to ignore/dismiss. Lack of knowledge of how to report. Difficult to identify abuse. Implement strategies to identify and monitor abusive individuals. Acknowledge responsibility of the facility to report an incident. Maintain a high level of sensitivity. Aggressively investigate any suspected abuse or neglect. 45 Restraints Restraints Must be in writing and for medical symptoms. May not be used for discipline or staff convenience. In emergency, nurse may apply restraints temporarily to protect resident or others. Documented Physician informed promptly 46 47 What is a restraint? Physical Chemical 48 145 07 Resident Rights Barriers to Resident Rights Observation Barriers to Resident Rights Observation Residents Lack awareness of rights violation. Unable to exert control. Fear assertiveness. Disease processes. Tolerate rights violation. Staff Lack of training and supervision. Cultural differences. Facility Staff training and supervision. Physical plant. Toleration of rights violation. 49 Common Situations Where Resident Rights are Relevant Most Common Violations 50 Confidentiality: 483.10(e) Privacy: 483.10(e) Independence: 483.15 Conflicts between individual and facility. Advance directives and refusal of medical care. Conflicting rights. Balance between protection and risk situations. 51 How Facilities Can Promote Resident Rights 52 Promotion of Resident Rights Knowledge of resident rights. Advocacy for residents. Promote resident rights to the staff. Aging Simulation Speaker series to residents, families, staff Posters Resident Rights Bingo (Available from Colorado State Ombudsman Program (303) 722-0300 ) 53 Awards Staff fantasy exercise Staff questionnaire Vignettes for staff 54 146 07 Resident Rights Attending Physician’s Tasks Attending Physician’s Tasks Providing the best possible care. Monitoring drug regimens. Eliminating restraints. Ensuring privacy. Resident advocacy. Ethical decision making. Decision making capacity. Advance directives. Resident’s right to refuse treatment. Communication 56 55 Medical Director’s Tasks Medical Director’s Tasks Overseeing Ensuring Quality of care Quality of life Quality improvement Policies Evaluation of decision making capacity. Determination of substitute decision makers. Access to quality care. Advance directives. Ethical decision making. 57 58 Medical Director’s Tasks Conclusion Advocating for resident rights. Ability of resident to voice concerns. Privacy Right to refuse treatment. Choice Respect Dignity 59 OBRA ’87 changed nursing home expectations by emphasizing determining and meeting the needs of nursing home residents. The Federal Resident Bill of Rights created by OBRA ’87 was aimed at improving residents quality of life. There are many barriers that interfere with residents rights in the nursing home. As attending physicians and medical directors we can help facilities ensure that residents rights are honored. 60 147 08a Financial Issues Learning Objectives 08a Financial Issues Long Term Care Financing Medical Directors Tasks in Organizational Budgeting Physician Billing, Coding, and Documentation Core Curriculum on Medical Direction 2 1 Financial Issues Explain the differences between the sources of long term care funding. Communicate effectively with the administrator concerning the expense and revenue aspects of the facility budget. Define the nature of the Medical Director’s functions and tasks relative to financial issues in long term care facilities. Identify issues related to documentation, coding, and physician reimbursement in long term care. Financial Overview and Long Term Care Funding Three main components to this module. 1. Financial Overview and Long Term Care Funding National Funding Data Nursing Home Payment Systems Accountable Care Organizations The Big Picture 2. Basic Accounting, the Nursing Home Budget, and the Medical Directors Tasks in Organizational Budgeting. 3. Physician Documentation, Coding and Billing in Long Term Care. 4 3 Distribution of Personal Health Care Expenditures by Source of Payment, 1999 and 2009 1999 Public 42.6% Private 57.4% 2009 Public 47.4% Private 52.6% Projected National Health Expenditures in the United States, by Source of Payment, 2010 Private Health Insurance Other Private Spending Medicare Out-of-Pocket Payments $1.1 Trillion $2.1 Trillion Notes: Personal health care expenditures are spending for health care services, excluding administration and net cost of insurance, public health activity, research, and structures and equipment. Out-of-pocket health insurance premiums paid by individuals are not included in Consumer Out-of-Pocket; they are counted as part of Private Health Insurance. Medicaid spending for the State Children's Health Insurance Program (which began in 1998) is included in Other Government Programs, not in Medicaid. Source: Kaiser Family Foundation calculations using NHE data from Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group, at http://www.cms.hhs.gov/NationalHealthExpendData/ (see Historical; National Health Expenditures by type of service and source of funds, CY 1960-2009; file nhe2009.zip). Other Public Spending Medicaid and CHIP1 Total National Health Expenditures, 2010 = $2.6 Trillion NOTES: 1Includes Children’s Health Insurance Program (CHIP) and Children’s Health Insurance Program expansion (Title XIX). Percentages do not sum to 100% due to rounding. SOURCE: Centers for Medicare & Medicaid Services, Office of the Actuary, Updated National Health Expenditure Projections 2009-2019, January 2011. 148 08a Financial Issues Medicare Spending as a % of Total Federal Spending, Fiscal Year 2010 Medicare Enrollment, 1966-2010 Nonelderly Disabled (Under Age 65) Elderly (Age 65 and Older) Number in millions: 37.6 28.5 25.0 4.4 5.4 7.3 7.0 6.7 45.4 46.1 47.0 7.5 20% 8.0 7.6 20% 3.3 15% 2.9 3.0 2.2 19.1 20.5 19.1 34.2 31.1 39.6 44.0 42.5 43.3 22.8 20.5 25.5 31.0 28.2 33.2 34.3 35.8 37.0 36.3 37.9 38.5 19% 39.0 8% 6% Total Federal Spending, FY2010 = $3.5 Trillion 1966 1970 1975 1980 1985 1990 1995 2000 2005 2006 2007 2008 2009 2010 NOTES: Numbers may not sum to total due to rounding. People with disabilities under age 65 were not eligible for Medicare prior to 1972. SOURCE: Centers for Medicare & Medicaid Services, Medicare Enrollment: Hospital Insurance and/or Supplemental Medical Insurance Programs for Total, Fee-for-Service and Managed Care Enrollees as of July 1, 2008: Selected Calendar Years 1966-2008; 2009-2010, HHS Budget in Brief, FY2011. NOTES: FY is fiscal year. 1Amount for Medicare includes offsetting premium receipts. 2Other category includes disaster costs and negative outlays for Troubled Asset Relief Program. SOURCE: Office of Management and Budget, FY2011 Budget, Summary Tables; February 2010. Medicare Benefit Payments, by Type of Service, 2010 and 2020 Part A Parts A and B Outpatient Prescription Drugs Hospital Inpatient Services 11% Hospital Outpatient 6% Services Part B Medicare’s Share of National Personal Health Expenditures, by Type of Service, 2010 Part D Outpatient Prescription Drugs 19% 27% 27% 8% Physician 13% Payments 6% 5% Skilled Other Services 12% Nursing Facilities 10% 4% 23% Home Health 12% 11% Medicare Advantage (Part C) Medicare Benefit Payments 2010 = $509 Billion 12% 5% Medicare Benefit Payments 2020 = $914 Billion NOTES: Totals do not include administrative expenses and are net of recoveries. Other Services include hospice services; durable medical equipment; ambulance services; independent, physician in-office, and hospital outpatient department laboratory services; hospital outpatient services that are not paid for using the prospective payment system (PPS); Part B prescription drugs; rural health clinic services; outpatient dialysis; and benefit payments not allocated to specific services, including adjustments to reflect year-to-date spending (2010), and savings from the Independent Payment Advisory Board (2020). SOURCE: Congressional Budget Office, Medicare Baseline, August 2010. Estimated Sources of Medicare Revenue, 2010 Expenditures in Billions Medicare Total $489 $31 $235 $62 $105 $29 $2,142 $77 $789 $260 $536 $149 NOTES: Total also includes dental care, durable medical equipment, other professional services, and other personal health care/products. SOURCE: Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Expenditure Projections 2009-2019, February 2010. Medicare Funding of Benefits Mandatory Enrollment Optional 25% 25% 2.9% divided between workers and employers TOTAL $499 billion PART A $218 billion PART B $219 billion PART D $63 billion SOURCE: 2010 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. 12 149 08a Financial Issues Historical and Projected Number of Medicare Beneficiaries and Number of Workers Per Beneficiary Number of Beneficiaries (in millions) Components of Average Health Care Spending by Medicare Households, 2009 Share of Total Spending Number of Workers Per Beneficiary 9.8% ($3,038) Other Household Spending 85.1% Health Care 14.9% 2.6% ($804) Medical Services (17.4%) 2.1% ($654) Prescription Drugs (14.2%) 0.4% ($125) Average Total Spending = $30,966 Health Insurance (65.7% of Health Care Spending) Medical Supplies (2.7%) Average Health Care Spending = $4,620 SOURCE: 2010 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds. NOTES: Numbers may not sum to total due to rounding. SOURCE: Kaiser Family Foundation analysis of the Bureau of Labor Statistics Consumer Expenditure Survey Interview and Expense Files, 2009. Characteristics of the Medicare Population Projected Medicare Outlays, 2010-2020 Percent of total Medicare population: Total Outlays in billions:* Income <200% FPL ($21,660 in 2010) 3+ Chronic Conditions Cognitive/Mental Impairment Fair/Poor Health Under-65 Disabled 2+ ADL Limitations Age 85+ Long-term Care Facility Resident NOTE: ADL is activity of daily living. SOURCE: Income data for 2009 from U.S. Census Bureau, Current Population Survey, 2009 Annual Social and Economic Supplement. All other data from Kaiser Family Foundation analysis of the Centers for Medicare & Medicaid Services Medicare Current Beneficiary 2008 Access to Care file. NOTE: Outlays have been rounded to the nearest whole number and exclude offsetting receipts SOURCE: Kaiser Family Foundation based on data from Congressional Budget Office, August 2010. Net Effect of Major Legislation on Medicare Spending Net Spending/Savings as a Share of Projected Medicare Spending Over 10 Years (1999) (2000) (2003) DRA MIPPA (2005) (2008) -$394 $25 $82 $391 -$23 -$2 -$424 $3.4 $3.2 $3.2 $3.9 $5.6 $6.8 $7.1 BBA 10-yr Medicare spending/savings (in $ billions): BBRA BIPA MMA Net savings (in $ trillions): Overall Medicare spending grew from $3.3 billion in 1967 to nearly $414 Billion in 2009. (2010) Net spending 10-yr Medicare baseline amounts Medicare Spending PPACA Dollars in Billions (1997) Source: Kaiser Family Foundation analysis of Congressional Budget Office (CBO) estimates. Notes: Shares are rounded to the nearest whole number. Net spending as a percent of baseline for MIPPA is rounded up from -0.02%; estimate for DRA is rounded from -0.47%. Baseline amounts are based on CBO projections of 10-year Medicare baseline spending prior to enactment of legislation. Note: Overall spending includes benefit dollars, administrative costs, and program integrity costs. Represents Federal spending only. Source: CMS, Office of the Actuary. 18 150 08a Financial Issues Growth in Medicare Skilled Nursing Facility Program Payments PAYMENT IN MILLIONS After rising rapidly during the 1990s, payments to skilled nursing facilities fell for the first time in 1999, then continued their rise. Implementation of Medicare Catastrophic Coverage Act of 1988 Medicaid Financials Transition To SNF PPS MCCA Repealed $9,617 $11,199 CALENDAR YEAR 20 19 Medicaid Has Many Vital Roles In Our Health Care System Assistance to Medicare Beneficiaries Health Insurance Coverage 29 million children & 15 million adults in low-income families; 15 million elderly and persons with disabilities Long-Term Care Assistance 8.9 million aged and disabled — 21% of Medicare beneficiaries Medicaid in the Health System, 2009 Medicaid as a share of national health care spending: 1 million nursing home residents; 2.8 million community-based residents MEDICAID Support for Health Care System and Safety-net State Capacity for Health Coverage 16% of national health spending; 40% of long-term care services Federal share can range from 50 - 83%; For FFY 2012, ranges from 50 - 74.2% Total National Spending (billions) $2,330 $759 $675 $137 $250 Note: Does not include spending on CHIP. SOURCE: Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group, National Health Expenditure Accounts, January 2011. SOURCE: Kaiser Commission on Medicaid and the Uninsured, 2011. Growth in Medicaid Long-Term Care Services Expenditures, FFY 1990-2009 Projected Spending on Health Care as a Percentage of Gross Domestic Product 25% Institutional Care Home and Community-Based Services 20% In Billions $92 $75 $54 $32 13% 87% 1990 32% $100 37% 41% 42% 70% 68% 63% 59% 58% 19.3% $122 15% 43% 30% 20% 80% $109 $115 10% Total National Health Spending 5% Medicare Spending 57% 3.4% Medicaid Spending 0% 2009 7.6% 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 $3,025 $3,225 $3,442 $3,684 $3,936 $4,204 $4,483 Total NHE: (in billions) 1995 2000 2002 2004 2006 2008 2009 Note: Home and community-based care includes home health, personal care services and home and community-based service waivers. Institutional care includes intermediate care facilities for the mentally retarded, nursing facilities, and mental health facilities. SOURCE: KCMU and Urban Institute analysis of HCFA/CMS-64 data. $2,472 $2,570 $2,703 $2,850 Source: Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group, at http://www.cms.hhs.gov/NationalHealthExpendData/03_NationalHealthAccountsProjected.asp#TopOfPage (see Projected; NHE Historical and projections, 1965-2019, file nhe65-19.zip). 151 08a Financial Issues Since The Start Of The Recession More Than 7 Million More Enrolled in Medicaid Medicaid-To-Medicare Provider Fee Ratios for All Services NH VT WA MT Monthly Enrollment in Millions 44.8 43.6 42.7 42.3 MN OR WI RI MI WY NE NV UT CO MO OK AZ NM OH IN ILIL KS CT NJ PA IA CA MA NY SD ID 50.3 48.7 46.9 ME ND WV AL DC SC AR MS MD NC TN TX DE VA KY GA LA AK FL HI Jun-07 Dec-07 Jun-08 Dec-08 Jun-09 Dec-09 Jun-10 U.S. Average = 72% of Medicare fees NOTE: Tennessee does not have a fee-for-service component in its Medicaid program SOURCE: S. Zuckerman, AF Williams, and KE Stockley, “Trends in Medicaid Physician Fees, 2003-2008,” Health Affairs, 28 April 2009. SOURCE: Analysis for KCMU by Health Management Associates, using compiled state Medicaid enrollment reports Statutory Federal Medical Assistance Percentages (FMAP), FY 2012 WA VT MT MN SD ID NV WI WY UT CO CA AZ NM PA IL KS OK TX MO IN WV KY MS AL VA CT NJ DE MD 28.7% ARRA Enhanced FMAP (2009-2011) 12.9% 12.7% DC 10.4% 9.9% 8.7% 8.4% SC 8.5% 5.5% GA FL 10.8% 10.1% 7.7% 7.6% 6.4% 4.9% 3.0% 3.8% 1.3% 5.8%5.7% 4.0% 50 percent (15 states) 51 – 59 percent (11 states) 60 – 66 percent (13 states) 67 – 74 percent (12 states including DC) 6.6% 7.3% 2.2% Enhanced FMAP / Federal Fiscal Relief (2003-2005) AK HI State RI NC TN AR LA OH Total NH MA NY MI IA NE Total and State Medicaid Spending Growth FY 2000 – FY 2012 ME ND OR < 70% (11 states including DC) 70-84% (7 states) 85-99% (21 states) 100%+ (11 states) -4.9% -10.9% 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 Adopted NOTE: State Fiscal Years. NOTE: Rates are rounded to nearest percent. These rates will be in effect Oct. 1, 2011 – Sept. 30, 2012. SOURCE: Federal Register,, Nov, 10, 2010 (Vol. 75, No. 217), pp. 69082-69083. http://edocket.access.gpo.gov/2010/pdf/2010-28319.pdf SOURCE: Historic Medicaid Growth Rates, KCMU Analysis of CMS Form 64 Data; FY 2008, 2009 and 2010, KCMU survey of Medicaid officials in 50 states and DC conducted by Health Management Associates, 2011 . Growth in Medicaid Expenditures and Enrollment Estimated Medicaid Enrollment in 2021 Under the House Budget Plan Enrollment in Millions ACA Repeal: -17M Total Cut: 36M Block Grant: -19M 75.9 M (48% Enrollment Cut) 39.5 M Current Law, Including ACA 29 House Budget Plan* *Assumes current enrollee spending growth and a reduction across all eligibility groups. Please see full report at http://www.kff.org/medicaid/8185.cfm. Source: Urban Institute estimates prepared for the Kaiser Commission on Medicaid and the Uninsured, May 2011. 152 08a Financial Issues Distribution of National Prescription Drug Expenditures by Source of Payment, 1999-2009 Medicaid Expenditures by Service, 2009 Home Health and Personal Care 14.4% Private Insurance DSH Payments Inpatient 4.8% 13.9% Physician/ Lab/ X-ray 3.7% Mental Health 1.2% Public Funds Long-Term Care 33.3% Outpatient/Clinic 7.1% ICF/MR 3.8% Drugs 4.3% Nursing Facilities 13.9% Consumer Out-ofPocket Payments Payments to Medicare 3.3% Acute Care 61.9% Other Acute 8.2% Payments to MCOs 21.4% Total = $366.5 billion Notes: Percentages may not total 100% due to rounding. NOTE: Total may not add to 100% due to rounding. Excludes administrative spending, adjustments and payments to the territories. SOURCE: Urban Institute estimates based on data from CMS (Form 64), prepared for the Kaiser Commission on Medicaid and the Uninsured. Source: Kaiser Family Foundation calculations using NHE data from Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group, at http://www.cms.hhs.gov/NationalHealthExpendData/ (see Historical; National Health Expenditures by type of service and source of funds, CY 1960-2009; file nhe2009.zip). Nursing Homes Total Funding CY 2009 Medicaid remains the largest single payer of nursing home care. Other Public Funds, 3% Out-of-pocket, 27% Medicaid, 42% Private Health Ins, 7% Medicare, 18% Private Funding 37% Other Private Funds, 3% Average Hospital, SNF, Hospice and Home Health Medicare Charges (FY 2006) Hospital charges/day $5,036 SNF charges/day $535 Hospice charges/day $144 Home health charges/visit $137 Annual Statistical Supplement, to the Social Security Bulletin, October 2008 Source: AAHSA, Health Advocacy Department 33 34 Long Term Care Funding Nursing Home Payment Systems Medicaid Historical Perspective 1960’s Federal law silent on how to set rates. Medicaid, Medicare, and Prospective Payment System There was no information available on costs to the nature of the “industry.” Very low unrealistic flat per diem rates set. “How Did We Get To This Place?” 35 36 153 08a Financial Issues Medicaid Boren Amendment Historical Perspective 1981 1972 Congress required payments to be related to costs. To meet legislative mandate and lack of data, - States Used Medicare Rates. “Reasonable and Adequate” Payments to facilities for efficiently and economically operated facilities included costs of complying with OBRA. 1980 Required states to make “Medicare cost based payments are inherently inflationary.” Development of the “cost plus” system. 37 38 Boren Amendment BBA 97 Repealed Boren Tremendous Litigation from Providers Against States 1990 Supreme court gave nursing homes the right to challenge state rates on: Substantive Issues Procedural Matters Replaced with existing “equal access” provision. Requires state Medicaid payment to be “consistent with efficient, economic, and quality care to enlist enough providers to have appropriate care available.” Less precise wording on quality - No OBRA. Changed procedures and providers rights. 39 Balanced Budget Act 1997 Medicare Influence on Medicare and Medicaid 40 Savings over 5 years: Medicare SNF $115 B $ 9.2B Medicaid $ 16B Evolution Past to Present 41 42 154 08a Financial Issues Benefits Benefits Part A - Coverage Part B - Coverage Inpatient Hospital Postacute Care in Nursing Home Physician Services Outpatient In-Pt 3 Days Within 30 Days of Admission Days 1 to 20 - No Deductible or Co-pay Days 21 to 100 - Co-pay Days > 100 - No Coverage Diagnostics PT, OT, Speech Audiology Some Ambulance Some Home Health Hospice 43 2012 Medicare Monthly Premium Hospital inpatient Days 1-60 Days 61-90 $1,156.00 deductible $289.00/day deductible First 20 days Days 21-100 Retrospective- “Cost Plus” Routine cost limits for overhead. no charge $144.50/ day deductible Part B $451 NH Medicare in NH: Old System Pre PPS Part A 44 Deductible Monthly premium $140.00 $110.50 Nursing Room and Board No limits on ancillary services. Part D Monthly Premium $ 30.00 (Approximate Basic) 45 46 Medicare in NH: Old System Pre PPS - Costs Changes to NH Industry Retrospective- “cost plus” Routine Hospital Prospective Payment System (DRG) 1983 Major change in financing mechanism. Introduced the concept of capitated rates on a large scale to the health care system. - Paid in Full Therapy, Drugs, Lab Capital - Cost Limits Room, board, nursing, minor medical supplies, medical and psychologic social services Ancillary - Paid in Full Land, Building, Equipment, Interest 47 48 155 08a Financial Issues Prospective Payment System for Nursing Homes PPS Recent Evolution of PPS OBRA 93 – Provision in Conference Report for HCFA to develop PPS by 10/1/95. FY96 – Republican Conference Bill- Vetoed by President Clinton, had provision for SNF PPS with bipartisan support. BBA 97 - Balanced Budget Act “BUBBA 97” 49 Medicare SNF Changes with BBA 97 50 Prospective Payment System (PPS) Prospective Payment System (PPS) Two important features: Consolidated Billing Case Mix Per Diem: Not Episodic Resource Based: Not By Diagnosis 51 Federal Rate Calculation 52 Consolidated Billing Based on FY 1995 Costs (implemented 1997) Basics, Promises, and Problems a. Hospital and freestanding facilities b. Gradual reduction to one rate locally phased in over a 4 year period. Adjustments a. Urban/rural b. Geographic – wage index c. Case-Mix from MDS / RUGS III 53 54 156 08a Financial Issues Basics Consolidated Billing Excluded Services Skilled nursing facilities must submit all Medicare claims for all of the services that its residents receive. Excluded services Physician Services PA / NP Qualified Psychologists Nurse Anesthetists Home Dialysis Supplies Erythropoeitin for Dialysis Hospice Ambulance Trip to SNF for Initial Admission 55 56 SNF Responsibility to Bill Ends When: Consolidated Billing Promises Admission to the hospital. Admission to another SNF. Receives services from home health. Outpatient services from Medicare that that are not pursuant to plan of care. Discharged Provides an essential foundation for PPS by bundling into a single facility rate virtually all of the services that the PPS payment is intended to capture. Eliminates potential for duplicative billing. 57 Consolidated Billing Promises Spares beneficiaries from incurring out-of-pocket expenses. Requires and enhances SNF’S capacity to meet responsibility to oversee and coordinate care. 58 Significance of Consolidated Billing to Facility 59 SNF is no longer able to unbundle services to outside suppliers. Increased accounting costs. Increase in responsibility of facility to control costs. Therapy Supplies 60 157 08a Financial Issues Consolidated Billing Problems Prior System Versus PPS Prior System Retrospective Cost Reimbursement New System P.P.S. Ancillaries- not capitated Incentive to shift costs to ancillary areas. More ancillary costmore shift. Grossly under- funded long term care. Rate based on RUGs. Ancillaries capitated Incentive to reduce costs and streamline. Less ancillary costmore profit. Average 18% cut in funding. Profit margins were no where near this level leading to wide spread bankruptcies in the industry. CMS response - “bad management” Congressional response FY 2000 (too late) - more money. 61 62 Case Mix Adjustments Hospice Financing Resource Utilization Groups (RUGs) Version III (RUGsIII) 44 (53) Group Classification Adjusts for resources used. Based on staff time measures. Classified from MDS Each group has different payment. 63 Home Health Prospective Payment Medicare “capped” hospice payment per patient to approximately $23,000 in 2009. A 2008 report by National Hospice and Palliative Care Organization showed that 84.3% of hospice care was paid for by Medicare. Hospice reduced Medicare costs by an average of $2,309 per hospice patient in 2008. In fiscal year 2008 - 09, 2% of hospice funding came from charitable contributions.. 64 Long Term Care Continuum Home Health Care: Payer Mix FY 2006 Medicaid-Federal only 19% Medicare 37% Other Public 3% Out-of-pocket 10% * State/Local Governments 19.9 % Private Insurance 12% Source: CMS, Office of the Actuary, National Health Statistics Group 65 Includes Home Health Care and Hospice *State/local government funding includes Medicaid matching funds 66 158 08a Financial Issues Home Health Care before and after PPS Implementation in October ‘02 Home Health Care before and after PPS Implementation in October ‘02 1997 2000 2008 Home Health Payments (billions) $17.7 $8.5 $16.9 Users (millions) 3.6 2.5 3.2 Number of Visits (millions) 258.2 90.6 177.8 Average home health outlays Average payment/client Visit Type (percent of total) Skilled Nursing Home Health Aid Therapy Social Services 41 48 10 1 49 31 19 1 55 18 26 1 $2,914 (1999) $3,803 (2002) $5,337 (2008) Average number of visits 97/client (1999) 37/client (2002) 38/client (2008) 67 Home Health Funding PPS began October 2002 68 The Impact of Prospective Payment Home Health 80 different payment levels. Varies by geographic area and county. Case-mix weighted reimbursement. Modeled after the PPS for skilled nursing. 69 Boling, PA. Using Home Care to Improve Outcomes and Lower Costs. Clinical Geriatrics:12;30-35, 2004 70 Basis Accounting and the Medical Directors Tasks in Organizational Budgeting As It Applies To The Nursing Home and Other Long Term Care Organizations 8.1 – 8.2 71 72 159 08a Financial Issues Basic Accounting Objectives Basic Accounting Concepts To understand basic accounting concepts. To be familiar with budgeting and the medical director’s tasks in the budgeting process. Revenue versus expense Asset versus liability Net worth Balance sheet Income statement Budget To be able to delineate the sources of revenue and the major expense items in long term care. To understand cost accounting and the role of cost containment. 73 74 Budgeting Budgeting Revenue Purpose - An attempt to predict revenue and expense for a specific period of time (usually one year). Need to consider: Expense Current financial status (balance sheet) Current financial performance (budget, income statement, and variances) Goals of organization (profit vs. service) 75 Basic Accounting Operational (supplies, insurance, payroll, food, utilities, etc.) Capital (depreciable items, such as machines, buildings, expensive computer 76 hard/software) Basic Accounting Asset - What you have that is to your financial benefit. Patient care (third party, private pay, long term care insurance) Investment Property Cash, securities Accounts receivable Net Worth - The equity you have in the business. Net Worth = Assets - Liabilities Liability - What you have that is to your financial detriment. Mortgage Accounts payable Depreciation 77 78 160 08a Financial Issues Basic Accounting Basic Accounting Income Statement - Measures the financial performance of the organization at a point in time, measuring assets, liabilities, and net worth. Budget - A prediction of future financial performance; it essentially predicts a future income statement. Capital Expense - One above an arbitrary defined cost, usually is non recurring and can be depreciated over a defined time period. Examples: Budget Variances - How the actual income statement compares with the budgeted (predicted) one; variances may be favorable or unfavorable. 79 Cash Versus Accrual Cost - Accounting Accounting Methods Cash - Exact amounts in and out. Accrual - Takes into consideration: “Accounts Receivable” (AR) “Accounts Payable” (AP) More accurate picture of status. A new rehabilitation section to the nursing home qualifies (non recurring, high cost, eligible for depreciation). A new employee does not count (recurring expense). A new coffee pot does not count (cost threshold not met). 80 Defining the cost of providing a given product or service (material, time, admin). An hour of nursing care. Continence care Providing ADA vs. “no concentrated sweets” diabetic diets. Critical information for surviving in capitated or prospective payment system as opposed to cost-plus reimbursements. 81 82 Cost Containment Cost Containment “Reduce overhead” Reducing expense to: Advantages Increase profit (or excess of revenue over expense) Decrease loss (or excess of expense over revenue) Unless system is poorly managed and full of “fat”, may lead to reduced quantity and quality of care. 83 Trim waste. Increase revenue for profit or services. Disadvantages Interfere with/reduce quality of care. Alienate staff by increasing workload. Alienate medical staff by care issues or by making milieu less “user-friendly.” 84 161 08a Financial Issues Dashboard for Revenues/Operations Resident Revenue Minus Resident Expense Divided by Resident Revenue 85 Total Operating Expenses Divided by Total Operating Revenues 86 Cash on Hand Required by Bond Covenant 87 Ability to Pay the Annual Debt Service 89 88 Occupancy History of a CCRC 90 162 08a Financial Issues History of Staffing Patterns Budgeting Steps for Medical Director for medical staff budget: Prepare medical staff objectives and goals. Consider prior years’ revenue and expense. Consider coming year’s revenue and expense. Justify budget requests. Focus on new items/programs/services. If budgeted expense is in excess of budgeted revenue, indicate how the shortfall will be made up. 91 92 Budget Medical Director’s Tasks Departmental budget Provide input on medical care issues. New treatments such as skin/wound care. Human resource needs. CQI data 8.3 – 8.6 93 Accountable Care Organizations 94 Challenges With Our Present System of Care 1. Lack of integration of our health care system. 2. System of reimbursement is based upon productivity. 3. Quality of care, cost of care, and patient satisfaction plays a minor role in providers’ salaries. 4. Increase cost of care for our dually eligible residents (Medicare and Medicaid). ACO 95 96 163 08a Financial Issues Why is There Such a Variation in Cost of Care in Our Country? Review the article, “The Cost Conundrum” written by Dr. Atul Gawande and published in the New Republic in June of 2009. How Will Quality of Future Health Care be Measured? Successfully meeting the preestablished Quality Measures. Delivering cost effective care. Demonstrating good patient satisfaction of their care. 97 98 Potential Financial Benefits of an ACO Key Components of an ACO Participate in a legal structure. Accurate reporting of Quality Measures. Requires a three year contract. Requires a minimum of 5000 patients. Requires an integrated clinical and administrative system. Patient centered care . 99 Examples of Cost Savings in a AMDA Medical Director’s Nursing Home Providers will share with the government savings depending on the level of risk. Savings are based on the expected cost of care in a geographic area verses the actual costs of care. The percentage of shared cost savings depends on the level of risk assumed in the ACO. 100 Readmission Rates During First 30 Days of Nursing Home Stay 180 160 140 120 100 Evercare Non-Evercare 80 60 40 20 0 2007 2008 2009 2010 2011 5 year history of admission rates/1000 residents/year (800 admissions/1000 residents/year is consider the average throughout the USA) 101 102 164 08a Financial Issues Preparing to Participate in an ACO Preparing to Participate in an ACO Establish a team charged with monitoring regulations and analysis of the impact on your organization. Review existing relationships with local hospitals and request a seat at the ACO table with the hospital(s). 103 Start collecting and sharing your nursing home data with hospitals and/or large physician groups. 1. Admissions and re-admissions to hospitals. 2. Number of ER visits per 1000 resident days. 3. Costs for common rehab care such joint replacements, CHF, strokes, etc. 104 Preparing to Participate in an ACO Develop a strategic or enhanced plans to handle post acute care patients such as: 1. Open or closed staff. 2. Staff rounding daily. 3. Limited number of providers. 4. Regular staff meetings. 5. JACHO approval. 105 165 08b Financial Issues - Coding Physician Payment Systems 08 Financial Issues Physician Billing, Coding, and Documentation Evaluation and Management Codes E/M Core Curriculum on Medical Direction 2 1 Historical Perspective Historical Perspective 1980’s – early 1990’s - Medicare Payment Policy for LTC Only one visit paid for q 30 days. Lower reimbursement if >1 patient seen on the same trip (but no reimbursement for travel to facility!) The net result of these short-sighted policies: Lower reimbursement in general- average $15. Large scale abandonment of LTC patients because of low reimbursement, “punishment” for seeing more than one patient, and only allowed to see patient once a month. When physician informed of a problem - “send to the ED.” No physician visits to the nursing home. Physicians abandon this practice site from their practice plans. They still went to the hospital, but not the nursing home across the street. 3 4 Medicare 8.7 – 8.14 5 6 166 08b Financial Issues - Coding Medicare Carriers Local insurance companies that contract with CMS to do Part B billing (some also do Part A) Physician Billing – Part B Medicare Carriers National Policy Broad guidelines, may be modified by LMRP (Local Medical Review Policy). 7 8 Medicare Carriers Medicare Carrier Manual LMRP – Local Medical Review Policy Individualized guidelines specific to each carrier. Generally follow AMA CPT descriptions. Generally follow CMS guidelines. 9 10 Medicare Claims Processing Manual, Pub.100-04 Goals for Session Chapter 12 – Physicians/Nonphysician Practitioners 1. Know what the rules are. 2. Know where the rules come from. 3. Know how to use the rules. 4. So that you can: 30.6 - Evaluation and Management Service Codes Get paid for what you do. 30.6.13 - Nursing Facility Services 11 General - Codes 99201 - 99499 Codes 99304 - 99318 12 167 08b Financial Issues - Coding Medicare Claims Processing Manual, Pub.100-04 SEC. 30.6.1 - Selection of Level of Evaluation and Management Service A. Use of CPT Codes Medicare Claims Processing Manual Manual http://new.cms.hhs.gov/manuals/downloads/clm104c12.pdf CMS Transmittal 808 (January 6, 2006) http://new.cms.hhs.gov/Transmittals/Downloads/R808CP.pdf Medlearn Matter Article http://new.cms.hhs.gov/MedlearnMattersArticles/downloads/M M4246.pdf Or go to cms.hhs.gov and look for Regulations and Guidance, Manuals 13 Medicare Claims Processing Manual, Pub.100-04, “Medical necessity of a service is the overarching criterion for payment in addition to the individual requirements of a CPT code.” “The volume of documentation should not be the primary influence upon which a specific level of service is billed. Documentation should support the level of service reported.” 14 Medicare Claims Processing Manual, Pub.100-04, 30.6.13 - Nursing Facility Services A. Visits to Perform the Initial Comprehensive Assessment and Annual Assessments B. Visits to Comply With Federal Regulations (42 CFR 483.40 (c) (1)) in the SNF and NF C. Visits by Qualified Nonphysician Practitioners 30.6.13 - Nursing Facility Services E. Incident to Services F. Use of the Prolonged Services Codes and Other Time-Related Services G. Gang Visits H. Split/Shared E/M Visit D. Medically Complex Care I. SNF/NF Discharge Day Management Service 15 16 Medicare Claims Processing Manual, Pub.100-04, Documentation Guidelines Do not Underdocument 30.6.13 - Nursing Facility Services B. Visits to Comply With Federal Regulations (42 CFR 483.40) Overall status of the patient Multiple diagnoses Co-morbidities Other complicating issues Family issues Facility issues 17 “Payment is made under the physician fee schedule by Medicare Part B for federally mandated visits. Following the initial visit by the physician, payment shall be made for federally mandated visits that monitor and evaluate residents at least once every 30 days for the first 90 days after admission and at least once every 60 days thereafter.” “Medicare Part B payment policy does not pay for additional E/M visits that may be required by State law for a facility admission or for other additional visits to satisfy facility or other administrative purposes.” “E/M visits, prior to and after the initial physician visit, that are reasonable and medically necessary to meet the medical needs of the individual patient (unrelated to any State requirement or administrative purpose) are payable under Medicare Part B.” 18 168 08b Financial Issues - Coding 30.6.13 C Medicare Claims Processing Manual, Pub.100-04, Visits by Qualified Nonphysician Practitioners 30.6.13 - Nursing Facility Services Medically Necessary Visits “Medically necessary E/M visits for the diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body member are payable under the physician fee schedule under Medicare Part B. CPT codes, Subsequent Nursing Facility Care, per day (99307 - 99310), shall be reported for these E/M visits even if the visits are provided prior to the initial visit by the physician.” State Regulations, State Scope of Practice All E/M visits shall be within the State scope of practice and licensure requirements where the visit is performed and all the requirements for physician collaboration and physician supervision shall be met when performed and reported by qualified NPPs. General physician supervision and employer billing requirements shall be met for PA services in addition to the PA meeting the State scope of practice and licensure requirements where the E/M visit is performed. 19 20 30.6.13 C 30.6.13 C Visits by Qualified Nonphysician Practitioners Visits by Qualified Nonphysician Practitioners Federally Mandated Visits SNF Federally Mandated Visits NF Following the initial visit by the physician, the physician may delegate alternate federally mandated physician visits to a qualified NPP who meets collaboration and physician supervision requirements and is licensed as such by the State and performing within the scope of practice in that State. 21 Per the regulations at 42 CFR 483.40 (f), a qualified NPP, who meets the collaboration and physician supervision requirements, the State scope of practice and licensure requirements, and who is not employed by the NF, may at the option of the State, perform the initial visit in a NF, and may perform any other federally mandated physician visit in a NF in addition to performing other medically necessary E/M visits. 22 30.6.13 I SNF/NF Discharge Day Management Requires a face-to-face visit. Reported for the date of the actual visit by the physician or qualified NPP even if the patient is discharged from the facility on a different calendar date. 99315-99316 Death may be reported using CPT code 99315 or 99316, depending on the code requirement, for a patient who has expired, but only if the physician or qualified NPP personally performed the death pronouncement. 23 Timing of Visits OBRA - NH Code / Regulation Every 30 days for first 90, then at least every 60 days. Medicare Will only pay for medically necessary and reasonable visits. 24 169 08b Financial Issues - Coding Timing of Visits Timing of Visits Medicare Acute care program. Does not recognize subacute care. Recent inclusions for some preventive type care. Medicare - Does not pay for routine or preventive care unless it is considered necessary and reasonable. No definition of medical necessity or what is considered reasonable. Influenza Pneumococcal vaccine “Initial / welcome” physical 25 26 Medical Necessity AMDA Definition “Evaluation and management services, diagnostic tests and procedures, treatments, medical/surgical procedures, equipment or supplies that in the judgment of the attending physician….are required to professionally assess, plan, manage and monitor the health care of a resident or patient in the facility within the parameters of generally accepted principles of medical practice.” AMDA White Paper, October 1999 8.15 – 8.20 27 28 CPT Codes For Long Term Care CPT Codes For Long Term Care Physician Reimbursement Relative Value System (RVU) Coding Documentation Guidelines Codes, Codes, and More Codes 29 30 170 08b Financial Issues - Coding CPT 2000-2003 2000 376,448 99301 539,609 99302 893,521 99303 99311 7,018,933 99312 7,766,568 99313 1,926,461 190,232 99315 65,497 99316 Total 18,777,349 Relative Value System Formula [(Work RVU x Work adjuster x Work GPCI) + (Practice Cost RVU x Practice Cost GPCI) + (Malpractice RVU x Malpractice GPCI)] x Conversion factor = PAYMENT 2002 2003 318,696 301,285 583,401 577,594 1,056,020 1,162,273 6,405,300 5,971,971 8,766,676 9,305,483 2,567,224 3,021,191 255,388 282,136 104,905 127,402 20,059,612 20,751,338 31 32 Resource Disk Documentation Guidelines Multiple Versions, 1995, 1997, proposed 2001 guidelines that were withdrawn. CPT Codes: The Evolution and Current State Continuing to be revised as we speak. Common sense CPT Coding for Hospice in Long Term Care JAMDA 2001 JAMDA 2004 Psychiatry Billing for Nursing Home Services JAMDA 2005 33 34 Evaluation and Management Codes (E/M) Evaluation and Management Codes (E/M) There are seven components to level of care, six of which are used in defining the level of service: History Examination Medical decision making Counseling Coordination of care Nature of the presenting illness Time 35 All evaluation and management codes have performance and documentation requirements. Your billing codes should reflect what evaluation and management was performed and documented. There are standards for each code in regard to: History taking Examination completeness Medical decision making Your notes should reflect the level of care you have performed. 36 171 08b Financial Issues - Coding NH CPT Codes – 1999-2006 AMA Documentation Guidelines 1999 - OLD Comprehensive Do not underdocument Overall status of the patient Multiple diagnoses Co-morbidities Other complicating issues Family issues Facility issues 2006 - NEW Initial 99315 99316 99307 99308 99309 99310 Discharge Services 99315 99316 99318 99304 99305 99306 Subsequent Care 99311 99312 99313 Discharge Services 99301 99302 99303 Subsequent Care Annual 37 Level Of E/M Service 38 Level of E/M Service How To Choose Level Of Service? History Examination Medical decision making 39 40 4 Types of History Level of E/M Service History Examination Problem Focused Expanded Problem Focused Detailed Comprehensive 3 Defined Components To Each Level Medical decision making History of Present Illness Review of Systems Past Family, Social History 41 42 172 08b Financial Issues - Coding Extent/Level of History Subsequent HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A Extended Extended Extended Com plete Extent/Level of History Initial HPI ROS PFSH TYPE 99307 Brief N/A N/A PROB. FOC EXP. P F 99308 Brief Prob. Pert. N/A EXP. P F Pertinent DET AILED 99309 Extended Extended Pertinent DET AILED 99304 Com plete COMP. 99310 Extended Com plete Com plete COMP. 99304-6 43 44 Extent/Level of History Subsequent History History Of Present Illness – 2 Types 1. Brief 1 to 3 Elements 2. Extended 4 Elements -OR Status of at least 3 chronic or inactive conditions. HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC 99307 Brief Prob. Pert. N/A EXP. P F 99308 Extended Extended Pertinent DETAILED 99309 Extended Complete Complete COMP. 99310 45 46 Extent/Level of History Initial History Review of Systems – 3 Types HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A EXP. P F Extended Extended Pertinent DETAILED 99304 Extended Complete Complete COMP. 99304-6 47 1. Problem Pertinent One system 2. Extended 2 to 9 systems 3. Complete At least 10 systems 48 173 08b Financial Issues - Coding Extent/Level of History Subsequent HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A Extended Extended Extended Complete Extent/Level of History Initial HPI ROS PFSH TYPE 99307 Brief N/A N/A PROB. FOC EXP. P F 99308 Brief Prob. Pert. N/A EXP. P F Pertinent DETAILED 99309 Extended Extended Pertinent DET AILED 99304 Complete COMP. 99310 Extended Com plete Com plete COMP. 99304-6 49 50 History Past, Family, and/or Social History – 2 Types Extent/Level of History Initial 1. Pertinent - 99304 2. Complete – 99304-6 Not required for subsequent nursing facility care. HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A EXP. P F Extended Extended Pertinent DET AILED 99304 Extended Com plete Com plete COMP. 99304-6 51 Level of E/M Service 52 Extent of Examination History Problem focused Examination Expanded problem focused Medical decision making Detailed Comprehensive 53 54 174 08b Financial Issues - Coding Extent of Examination Level of E/M Service Initial Codes Detailed 99304 (Or comprehensive) Comprehensive (full) exam 99305-99306 Subsequent Codes 2 of 3 key components Appropriate exam Geriatric exam History Examination Medical decision making 55 56 Medical Decision Making Not Well Defined Components Except For Risk Medical Decision Making 4 Levels of Complexity Straightforward Low Moderate High Number of diagnoses / management options Amount / complexity of data Risk of complications, morbidity, mortality 57 Complexity Of Medical Decision Making – Subsequent (2 of Three) # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Multiple Ex te nsive Lim ite d Mode ra te Ex te nsive Low Mode ra te High LOW MODER ATE HIGH 58 Complexity Of Medical Decision Making – Initial (3 of Three) # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99304 Lim ite d Lim ite d Low LOW 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 Ex te nsive Ex te nsive High HIGH 99308 99309 99310 59 9930660 175 08b Financial Issues - Coding Medical Decision Making Medical Decision Making Number Of Diagnoses / Management Options – 4 Types Amount / Complexity Of Data – 4 Types 1. Minimal 1. Minimal or none 2. Limited 2. Limited 3. Multiple 3. Moderate 4. Extensive 4. Extensive 61 Medical Decision Making Complexity Of Medical Decision Making – Subsequent (2 of Three) Risk Of Complications, Morbidity, Mortality - 4 Types # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Lim ite d Low LOW 99308 Multiple Mode ra te Mode ra te MODER ATE 99309 Ex te nsive Ex te nsive High HIGH 99310 1. Minimal 62 1 self limited / minor problem No meds, minimal lab 2. Low 2 or more self limited or minor problems One stable chronic illness Acute uncomplicated illness OTC Meds, PT, OT 63 Complexity Of Medical Decision Making – Initial (3 of Three) # DIAG AMT DATA RISK TYPE 64 Medical Decision Making Risk Of Complications, Morbidity, Mortality CODE Minim a l Minim a l Minim a l STR AIGHT. 99304 Lim ite d Lim ite d Low LOW 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 Ex te nsive Ex te nsive High HIGH 3. Moderate 9930665 1 or more chronic illness w/ mild exacerbation. 2 or more stable chronic prob. Acute illness with systemic symp. Undiagnosed new problem with uncertain prognosis. Prescription meds 66 176 08b Financial Issues - Coding Complexity Of Medical Decision Making – Subsequent (2 of Three) # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Lim ite d Multiple Mode ra te Ex te nsive Ex te nsive Low Mode ra te High LOW MODER ATE HIGH Complexity Of Medical Decision Making – Initial (3 of Three) AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99304 Lim ite d Lim ite d Low LOW 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 Ex te nsive Ex te nsive High HIGH 99308 99309 99310 67 Medical Decision Making 9930668 Complexity Of Medical Decision Making – Subsequent (2 of Three) Risk Of Complications, Morbidity, Mortality # DIAG # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Lim ite d Low LOW 99308 Multiple Mode ra te Mode ra te MODER ATE 99309 Ex te nsive Ex te nsive High HIGH 99310 4. High 1 or more chronic illnesses w/ severe exacerbation. Acute or chronic illnesses that pose a threat to life. Abrupt change in neuro status. Surgery, parenteral meds, DNR 69 Complexity Of Medical Decision Making – Initial (3 of Three) 70 NH CPT Codes – 1999-2006 AMA # DIAG AMT DATA RISK TYPE CODE 1999 - OLD Comprehensive Minim a l Minim a l Minim a l STR AIGHT. 99304 Lim ite d Lim ite d Low LOW 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 99315 99316 Ex te nsive High HIGH 9930671 99304 99305 99306 Subsequent Care 99307 99308 99309 99310 Discharge Services 99315 99316 99318 Ex te nsive 2006 - NEW Initial 99311 99312 99313 Discharge Services 99301 99302 99303 Subsequent Care Annual 72 177 08b Financial Issues - Coding Extent/Level Of History Initial Initial Nursing Facility Care 99304 (3 of three) HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A EXP. P F Extended Extended Pertinent DET AILED 99304 Extended Com plete Com plete COMP. 99304-6 Detailed or comprehensive HX Detailed or comprehensive exam Medical decision making: Straightforward / low Used for: Initial admission / readmission Usually, the problem(s) requiring admission are of low severity. 73 74 Complexity Of Medical Decision Making – Initial (3 Of Three) Initial Nursing Facility Care # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99304 Lim ite d Lim ite d Low LOW 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 99305 (3 of three) Comprehensive HX Comprehensive exam Medical decision making: Used for Ex te nsive Ex te nsive High HIGH 9930675 Moderate Initial admission / readmission Usually, the problem(s) requiring admission are of moderate severity. 76 Complexity Of Medical Decision Making – Initial (3 of Three) Extent/Level Of History Initial # DIAG AMT DATA RISK TYPE CODE PROB. FOC Minim a l Minim a l Minim a l STR AIGHT. 99304 N/A EXP. P F Lim ite d Lim ite d Low LOW 99304 Extended Pertinent DET AILED 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 Com plete Com plete COMP. 99304-6 Ex te nsive Ex te nsive High HIGH HPI ROS PFSH TYPE Brief N/A N/A Brief Prob. Pert. Extended Extended 77 9930678 178 08b Financial Issues - Coding Extent/Level Of History Initial Initial Nursing Facility Care 99306 (3 of Three) Comprehensive HX Comprehensive exam Medical decision making: High Used for: Initial admission / readmission Usually, the problem(s) requiring admission are of high severity. HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A EXP. P F Extended Extended Pertinent DET AILED 99304 Extended Com plete Com plete COMP. 99304-6 79 80 Complexity Of Medical Decision Making – Initial (3 of Three) Subsequent Care # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99304 Lim ite d Lim ite d Low LOW 99304 Multiple Mode ra te Mode ra te MODER ATE 99305 Ex te nsive Ex te nsive High HIGH 9930681 Extent/Level Of History Subsequent 99307 (2 of three) Problem focused HX Problem focused exam Medical decision making: Straightforward Used for Patient stable, recovering, or improving “Routine / regulatory” visit 82 Complexity Of Medical Decision Making – Subsequent (2 of Three) # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Lim ite d Low LOW 99308 99309 Multiple Mode ra te Mode ra te MODER ATE 99309 99310 Ex te nsive Ex te nsive High HIGH 99310 HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC 99307 Brief Prob. Pert. N/A EXP. P F 99308 Extended Extended Pertinent DET AILED Extended Com plete Com plete COMP. 83 84 179 08b Financial Issues - Coding Extent/Level Of History Subsequent Subsequent Care 99308 (2 of Three) Expanded problem focused HX Expanded problem focused exam Medical decision making: Low Used for: Patient responding inadequately to RX or developed minor complication 85 “Routine / regulatory” visit HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC 99307 Brief Prob. Pert. N/A EXP. P F 99308 Extended Extended Pertinent DET AILED 99309 Extended Com plete Com plete COMP. 99310 86 Complexity Of Medical Decision Making – Subsequent (2 of Three) # DIAG AMT DATA RISK TYPE Subsequent Care CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Lim ite d Low LOW 99308 Multiple Mode ra te Mode ra te MODER ATE 99309 Ex te nsive Ex te nsive High HIGH 99310 87 Extent/Level Of History Subsequent 99309 (2 of three) Detailed HX Detailed exam Medical decision making: Moderate Used for Patient developed significant complication or significant new problem “Routine / regulatory” visit 88 Complexity Of Medical Decision Making – Subsequent (2 of Three) # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99307 Lim ite d Lim ite d Low LOW 99308 99309 Multiple Mode ra te Mode ra te MODER ATE 99309 99310 Ex te nsive Ex te nsive High HIGH 99310 HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC 99307 Brief Prob. Pert. N/A EXP. P F 99308 Extended Extended Pertinent DET AILED Extended Com plete Com plete COMP. 89 90 180 08b Financial Issues - Coding Extent/Level Of History Subsequent Subsequent Care 99310 (two of three) Comprehensive HX Comprehensive exam Medical decision making: High Used for The patient may be unstable or may have developed a significant new problem requiring immediate physician attention. HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC 99307 Brief Prob. Pert. N/A EXP. P F 99308 Extended Extended Pertinent DET AILED 99309 Extended Com plete Com plete COMP. 99310 91 92 Complexity Of Medical Decision Making – Subsequent (2 of Three) Discharge Services # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. 99315 - 30 minutes or less 99316 - More than 30 minutes 99307 Lim ite d Lim ite d Low LOW 99308 Used for: Multiple Mode ra te Mode ra te MODER ATE 99309 Ex te nsive Ex te nsive High HIGH 99310 93 Total Duration Of Time Final exam Instructions for continuing care Preparation of discharge records Prescriptions Referral forms 94 Extent/Level Of History – Annual Annual Visit 99318 (3 of three) Detailed interval HX Comprehensive exam Medical decision making: Low to moderate Used for Annual exam Usually, the patient is stable, recovering, or improving. 95 HPI ROS PFSH TYPE Brief N/A N/A PROB. FOC Brief Prob. Pert. N/A EXP. P F Extended Extended Pertinent DET AILED Extended Com plete Com plete COMP. 99318 96 181 08b Financial Issues - Coding Complexity Of Medical Decision Making – Annual # DIAG AMT DATA RISK TYPE CODE Minim a l Minim a l Minim a l STR AIGHT. Lim ite d Lim ite d Low LOW 99318 Multiple Mode ra te Mode ra te MODER ATE 99318 8.21 – 8.49 Ex te nsive Ex te nsive High HIGH 97 98 Financial Issues Long Term Care Financing Medical Directors Tasks in Organizational Budgeting Physician Billing, Coding, and Documentation 99 182